Targeting of antithrombin in hemophilia A or B with investigational siRNA therapeutic fitusiran-Results of the phase 1 inhibitor cohort
- PMID: 33587824
- PMCID: PMC8251589
- DOI: 10.1111/jth.15270
Targeting of antithrombin in hemophilia A or B with investigational siRNA therapeutic fitusiran-Results of the phase 1 inhibitor cohort
Abstract
Background: Fitusiran, an investigational small interfering RNA therapy, reduces antithrombin production to rebalance hemostasis in people with hemophilia A or B, with or without inhibitors.
Objectives: To evaluate the safety and efficacy of fitusiran treatment for people with moderate/severe hemophilia A or B with inhibitors.
Patients/methods: In this open-label phase 1, part D study, 17 males with hemophilia A or B with inhibitors received three once-monthly subcutaneous injections of fitusiran 50 mg (n = 6) or 80 mg (n = 11); followed for up to 112 days. Endpoints included safety (primary), pharmacokinetics/pharmacodynamics (secondary), annualized bleeding rate, and patient-reported outcomes (exploratory).
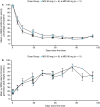
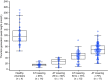
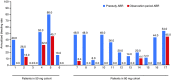
Results: The most common adverse event was injection site erythema (n = 8). No thrombotic events were reported. At nadir, mean (standard error of the mean [SEM]) antithrombin activity decreased from baseline by 82.0% (2.2) and 87.4% (0.7) in the 50 mg and 80 mg groups, respectively. Antithrombin reduction was associated with increased thrombin generation. 11/17 (64.7%) participants had no bleeds during the observation period (mean [standard deviation] 69.4 [16.3] days). Mean (SEM) changes from baseline in Haemophilia Quality of Life Questionnaire for Adults total (-9.2 [2.9]) and physical health (-12.3 [3.9]) domain scores suggested clinically meaningful improvement.
Conclusions: Monthly fitusiran was generally well tolerated, lowered antithrombin levels from baseline, and resulted in improved thrombin generation. These preliminary results suggest that monthly fitusiran treatment may reduce bleeding episodes and improve quality of life in participants with hemophilia A or B with inhibitors.
Keywords: antithrombin; fitusiran; hemophilia; inhibitors; siRNA.
© 2021 Sanofi. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
K. J. Pasi received grants, personal fees, and nonfinancial support from Sanofi Genzyme and BioMarin; personal fees and nonfinancial support from Hoffmann‐La Roche, OctaPharma, Catalyst Biosciences, Pfizer, Novo Nordisk, Swedish Orphan Biovitrum AB, Takeda, Biotest AG, and Sigilon Therapeutics; grants from Uniqure NV; and personal fees from ApcinteX. T. Lissitchkov has nothing to disclose. V. Mamonov received fees from Alnylam Pharmaceuticals and Sanofi Genzyme for serving as the principal investigator on clinical trials. T. Mant is an employee of Iqvia who received fees from Alnylam Pharmaceuticals and Sanofi Genzyme for conduct of the study. Outside the submitted work, P. Chowdary received research funding and fees for attendance at advisory board meetings from Pfizer, Bayer AG, CSL Behring, Freeline, Novo Nordisk, Swedish Orphan Biovitrum AB; personal fees from BioMarin and Uniqure NV; and fees for attendance at advisory board meetings from Chugai, Hoffmann‐La Roche, Takeda, Sanofi Genzyme, and Spark Therapeutics. L. Gercheva‐Kyuchukova received research grants from Alnylam Pharmaceuticals, Baxter, Bayer AG, Celgene, Gilead, and Novartis; honoraria and consulting fees from AbbVie, Amgen, Hoffmann‐La Roche, Johnson & Johnson, Novartis, Pfizer, and Takeda; and travel support from Celgene. K. Madigan was an employee of Alnylam Pharmaceuticals at the time of the study and is now an employee of Syros Pharmaceutical, Inc. H. Van Nguyen is an employee of Alnylam Pharmaceuticals. Q. Yu was an employee of Sanofi at the time of the study and is now an employee of Albireo Pharma Inc. B. Mei and C. C. Benson are employees of Sanofi Genzyme. M. V. Ragni received research funding and fees for attending advisory board meetings from Alnylam Pharmaceuticals, Sanofi Genzyme, BioMarin, Bioverativ, and Spark Therapeutics; research funding from CSL Behring, Genentech‐Roche, and Sangamo; and the gift of trial drug and fees for attending advisory boards from Baxalta/Takeda. M. Timofeeva has nothing to disclose. C. Bagot has nothing to disclose. P. Georgiev has nothing to declare.
Figures
References
-
- Monroe DM, Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol. 2006;26:41‐48. - PubMed
-
- Bolton‐Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361:1801‐1809. - PubMed
-
- Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388:187‐197. - PubMed
-
- Pipe SW, Valentino LA. Optimizing outcomes for patients with severe haemophilia a. Haemophilia. 2007;13(Suppl 4):1‐16.quiz 13 p following 16. - PubMed
-
- Ljung R, Gretenkort AN. The current status of prophylactic replacement therapy in children and adults with haemophilia. Br J Haematol. 2015;169:777‐786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

