Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment
- PMID: 33587887
- PMCID: PMC7906643
- DOI: 10.1016/S0140-6736(21)00306-8
Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment
Abstract
The COVID-19 pandemic is unlikely to end until there is global roll-out of vaccines that protect against severe disease and preferably drive herd immunity. Regulators in numerous countries have authorised or approved COVID-19 vaccines for human use, with more expected to be licensed in 2021. Yet having licensed vaccines is not enough to achieve global control of COVID-19: they also need to be produced at scale, priced affordably, allocated globally so that they are available where needed, and widely deployed in local communities. In this Health Policy paper, we review potential challenges to success in each of these dimensions and discuss policy implications. To guide our review, we developed a dashboard to highlight key characteristics of 26 leading vaccine candidates, including efficacy levels, dosing regimens, storage requirements, prices, production capacities in 2021, and stocks reserved for low-income and middle-income countries. We use a traffic-light system to signal the potential contributions of each candidate to achieving global vaccine immunity, highlighting important trade-offs that policy makers need to consider when developing and implementing vaccination programmes. Although specific datapoints are subject to change as the pandemic response progresses, the dashboard will continue to provide a useful lens through which to analyse the key issues affecting the use of COVID-19 vaccines. We also present original data from a 32-country survey (n=26 758) on potential acceptance of COVID-19 vaccines, conducted from October to December, 2020. Vaccine acceptance was highest in Vietnam (98%), India (91%), China (91%), Denmark (87%), and South Korea (87%), and lowest in Serbia (38%), Croatia (41%), France (44%), Lebanon (44%), and Paraguay (51%).
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures

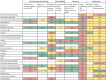
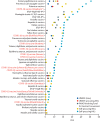
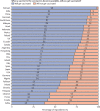
References
-
- International Monetary Fund A crisis like no other, an uncertain recovery. June, 2020. https://www.imf.org/en/Publications/WEO/Issues/2020/06/24/WEOUpdateJune2020
-
- WHO List of stringent regulatory authorities (SRAs) June 20, 2020. http://www.who.int/medicines/regulation/sras/en/
-
- WHO COVAX announces additional deals to access promising COVID-19 vaccine candidates; plans global rollout starting Q1 2021. Dec 18, 2020. https://www.who.int/news/item/18-12-2020-covax-announces-additional-deal...
-
- WHO Target product profiles for COVID-19 vaccines, version 3. April 29, 2020. https://www.who.int/publications/m/item/who-target-product-profiles-for-...
Uncited Reference
-
- Baric RS. Emergence of a Highly Fit SARS-CoV-2 Variant. N Engl J Med. 2020;383:2684–2686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

