Microglia-derived interleukin-10 accelerates post-intracerebral hemorrhage hematoma clearance by regulating CD36
- PMID: 33588074
- PMCID: PMC8058329
- DOI: 10.1016/j.bbi.2021.02.001
Microglia-derived interleukin-10 accelerates post-intracerebral hemorrhage hematoma clearance by regulating CD36
Abstract
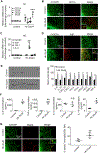
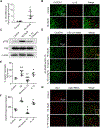
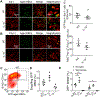
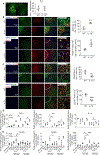
Hematoma size after intracerebral hemorrhage (ICH) significantly affects patient outcome. However, our knowledge of endogenous mechanisms that underlie hematoma clearance and the potential role of the anti-inflammatory cytokine interleukin-10 (IL-10) is limited. Using organotypic hippocampal slice cultures and a collagenase-induced ICH mouse model, we investigated the role of microglial IL-10 in phagocytosis ex vivo and hematoma clearance in vivo. In slice culture, exposure to hemoglobin induced IL-10 expression in microglia and enhanced phagocytosis that depended on IL-10-regulated expression of CD36. Following ICH, IL-10-deficient mice had more severe neuroinflammation, brain edema, iron deposition, and neurologic deficits associated with delayed hematoma clearance. Intranasal administration of recombinant IL-10 accelerated hematoma clearance and improved neurologic function. Additionally, IL-10-deficient mice had weakened in vivo phagocytic ability owing to decreased expression of microglial CD36. Moreover, loss of IL-10 significantly increased monocyte-derived macrophage infiltration and enhanced brain inflammation in vivo. These results indicate that IL-10 regulates microglial phagocytosis and monocyte-derived macrophage infiltration after ICH and that CD36 is a key phagocytosis effector regulated by IL-10. Leveraging the innate immune response to ICH by augmenting IL-10 signaling may provide a useful strategy for accelerating hematoma clearance and improving neurologic outcome in clinical translation studies.
Keywords: Hematoma clearance; IL-10; Intracerebral hemorrhage; Macrophage; Microglia.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Awad IA, Polster SP, Carrion-Penagos J, Thompson RE, Cao Y, Stadnik A, Money PL, Fam MD, Koskimaki J, Girard R, Lane K, McBee N, Ziai W, Hao Y, Dodd R, Carlson AP, Camarata PJ, Caron JL, Harrigan MR, Gregson BA, Mendelow AD, Zuccarello M, Hanley DF and M. I. T. Investigators (2019). Surgical Performance Determines Functional Outcome Benefit in the Minimally Invasive Surgery Plus Recombinant Tissue Plasminogen Activator for Intracerebral Hemorrhage Evacuation (MISTIE) Procedure. Neurosurgery 84(6): 1157–1168. - PMC - PubMed
-
- Bian Z, Shi L, Guo YL, Lv Z, Tang C, Niu S, Tremblay A, Venkataramani M, Culpepper C, Li L, Zhou Z, Mansour A, Zhang Y, Gewirtz A, Kidder K, Zen K, Liu Y, 2016. Cd47-Sirpalpha interaction and IL-10 constrain inflammation-induced macrophage phagocytosis of healthy self-cells. Proc. Natl. Acad. Sci. U.S.A 113 (37), E5434–E5443. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

