Quantitative three-dimensional collagen orientation analysis of human meniscus posterior horn in health and osteoarthritis using micro-computed tomography
- PMID: 33588085
- PMCID: PMC7610734
- DOI: 10.1016/j.joca.2021.01.009
Quantitative three-dimensional collagen orientation analysis of human meniscus posterior horn in health and osteoarthritis using micro-computed tomography
Abstract
Objective: Knee osteoarthritis (OA) is associated with meniscal degeneration that may involve disorganization of the meniscal collagen fiber network. Our aims were to quantitatively analyze the microstructural organization of human meniscus samples in 3D using micro-computed tomography (μCT), and to compare the local microstructural organization between OA and donor samples.
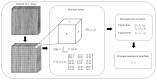
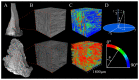
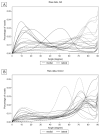
Method: We collected posterior horns of both medial and lateral human menisci from 10 end-stage medial compartment knee OA patients undergoing total knee replacement (medial & lateral OA) and 10 deceased donors without knee OA (medial & lateral donor). Posterior horns were dissected and fixed in formalin, dehydrated in ascending ethanol concentrations, treated with hexamethyldisilazane (HMDS), and imaged with μCT. We performed local orientation analysis of collagenous microstructure in 3D by calculating structure tensors from greyscale gradients within selected integration window to determine the polar angle for each voxel.
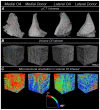
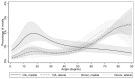
Results: In donor samples, meniscus bundles were aligned circumferentially around the inner border of meniscus. In medial OA menisci, the organized structure of collagen network was lost, and main orientation was shifted away from the circumferential alignment. Quantitatively, medial OA menisci had the lowest mean orientation angle compared to all groups, -24° (95%CI -31 to -18) vs medial donor and -25° (95%CI -34 to -15) vs lateral OA.
Conclusions: HMDS-based μCT imaging enabled quantitative analysis of meniscal collagen fiber bundles and their orientations in 3D. In human medial OA menisci, the collagen disorganization was profound with overall lower orientation angles, suggesting collagenous microstructure disorganization as an important part of meniscus degradation.
Keywords: Collagen organization; Contrast agent free micro-computed tomography; Meniscus microstructure; Osteoarthritis; Structure tensors.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
AT works as an associate editor (statistics) in Osteoarthritis and Cartilage.
ME has received grants from European Research Council, The Swedish Research Council, the Foundation for Research in Rheumatology, the Greta and Johan Kock Foundation, the Swedish Rheumatism Association, the Österlund Foundation, the Governmental Funding of Clinical Research program within the National Health Service in Sweden, and the Faculty of Medicine, Lund University, Sweden.
ME reports in 2019 serving on an advisory board for Pfizer (Tanezumab).
SS has received grants from Foundation for Research in Rheumatology and European Research Council.
Other authors (VK, IK, MF, EF, PÖ, VH, and JT) report no conflicts of interest.
Figures
References
-
- Kelly MA, Fithian DC, Chern KY, Mow VC. Biomechanics of Diarthrodial Joints. Springer; New York: 1990. Structure and Function of the Meniscus: Basic and Clinical Implications; pp. 191–211. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

