A Stronger Innate Immune Response During Hyperacute Human Immunodeficiency Virus Type 1 (HIV-1) Infection Is Associated With Acute Retroviral Syndrome
- PMID: 33588436
- PMCID: PMC8423478
- DOI: 10.1093/cid/ciab139
A Stronger Innate Immune Response During Hyperacute Human Immunodeficiency Virus Type 1 (HIV-1) Infection Is Associated With Acute Retroviral Syndrome
Abstract
Background: Acute retroviral syndrome (ARS) is associated with human immunodeficiency virus type 1 (HIV-1) subtype and disease progression, but the underlying immunopathological pathways are poorly understood. We aimed to elucidate associations between innate immune responses during hyperacute HIV-1 infection (hAHI) and ARS.
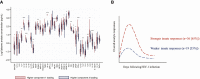
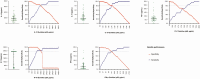
Methods: Plasma samples obtained from volunteers (≥18.0 years) before and during hAHI, defined as HIV-1 antibody negative and RNA or p24 antigen positive, from Kenya, Rwanda, Uganda, Zambia, and Sweden were analyzed. Forty soluble innate immune markers were measured using multiplexed assays. Immune responses were differentiated into volunteers with stronger and comparatively weaker responses using principal component analysis. Presence or absence of ARS was defined based on 11 symptoms using latent class analysis. Logistic regression was used to determine associations between immune responses and ARS.
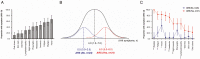
Results: Of 55 volunteers, 31 (56%) had ARS. Volunteers with stronger immune responses (n = 36 [65%]) had increased odds of ARS which was independent of HIV-1 subtype, age, and risk group (adjusted odds ratio, 7.1 [95% confidence interval {CI}: 1.7-28.8], P = .003). Interferon gamma-induced protein (IP)-10 was 14-fold higher during hAHI, elevated in 7 of the 11 symptoms and independently associated with ARS. IP-10 threshold >466.0 pg/mL differentiated stronger immune responses with a sensitivity of 84.2% (95% CI: 60.4-96.6) and specificity of 100.0% (95% CI]: 90.3-100.0).
Conclusions: A stronger innate immune response during hAHI was associated with ARS. Plasma IP-10 may be a candidate biomarker of stronger innate immunity. Our findings provide further insights on innate immune responses in regulating ARS and may inform the design of vaccine candidates harnessing innate immunity.
Keywords: HIV-1; IP-10; acute infection; acute retroviral syndrome; immune responses.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



References
-
- Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17:1871–9. - PubMed
-
- Braun DL, Kouyos RD, Balmer B, Grube C, Weber R, Günthard HF. Frequency and spectrum of unexpected clinical manifestations of primary HIV-1 infection. Clin Infect Dis 2015; 61:1013–21. - PubMed
-
- Tindall B, Barker S, Donovan B, et al. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Arch Intern Med 1988; 148:945–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

