Impact of treating iron deficiency, diagnosed according to hepcidin quantification, on outcomes after a prolonged ICU stay compared to standard care: a multicenter, randomized, single-blinded trial
- PMID: 33588893
- PMCID: PMC7885380
- DOI: 10.1186/s13054-020-03430-3
Impact of treating iron deficiency, diagnosed according to hepcidin quantification, on outcomes after a prolonged ICU stay compared to standard care: a multicenter, randomized, single-blinded trial
Abstract
Background: Anemia is a significant problem in patients on ICU. Its commonest cause, iron deficiency (ID), is difficult to diagnose in the context of inflammation. Hepcidin is a new marker of ID. We aimed to assess whether hepcidin levels would accurately guide treatment of ID in critically ill anemic patients after a prolonged ICU stay and affect the post-ICU outcomes.
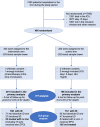
Methods: In a controlled, single-blinded, multicenter study, anemic (WHO definition) critically ill patients with an ICU stay ≥ 5 days were randomized when discharge was expected to either intervention by hepcidin treatment protocol or control. In the intervention arm, patients were treated with intravenous iron (1 g of ferric carboxymaltose) when hepcidin was < 20 μg/l and with intravenous iron and erythropoietin for 20 ≤ hepcidin < 41 μg/l. Control patients were treated according to standard care (hepcidin quantification remained blinded). Primary endpoint was the number of days spent in hospital 90 days after ICU discharge (post-ICU LOS). Secondary endpoints were day 15 anemia, day 30 fatigue, day 90 mortality and 1-year survival.
Results: Of 405 randomized patients, 399 were analyzed (201 in intervention and 198 in control arm). A total of 220 patients (55%) had ID at discharge (i.e., a hepcidin < 41 μg/l). Primary endpoint was not different (medians (IQR) post-ICU LOS 33(13;90) vs. 33(11;90) days for intervention and control, respectively, median difference - 1(- 3;1) days, p = 0.78). D90 mortality was significantly lower in intervention arm (16(8%) vs 33(16.6%) deaths, absolute risk difference - 8.7 (- 15.1 to - 2.3)%, p = 0.008, OR 95% IC, 0.46, 0.22-0.94, p = 0.035), and one-year survival was improved (p = 0.04).
Conclusion: Treatment of ID diagnosed according to hepcidin levels did not reduce the post-ICU LOS, but was associated with a significant reduction in D90 mortality and with improved 1-year survival in critically ill patients about to be discharged after a prolonged stay.
Trial registration: www.clinicaltrial.gov NCT02276690 (October 28, 2014; retrospectively registered).
Keywords: Anemia; Critically ill; Erythropoietin; Hepcidin; Iron (treatment); Iron deficiency; Length of stay; Mortality.
Conflict of interest statement
Sigismond Lasocki has received speaker honoraria from VIFOR Pharma, MASIMO, LFB and fee as member of advisory board and/or steering committee from VIFOR Pharma and Pfizer.. SLa is the coordinator of a multicenter randomized controlled trial on iron and tranexamic acid in hip fractured patients (HIFIT study, NCT02972294), for which PHARMACOSMOS gives iron for free. Philippe Seguin has received fee from LFB. None of the other authors have a competing interest to declare.
Figures

Comment in
-
Iron Deficiency Defined by Hepcidin in Critically Ill Patients.Crit Care. 2021 Apr 12;25(1):138. doi: 10.1186/s13054-021-03542-4. Crit Care. 2021. PMID: 33845879 Free PMC article. No abstract available.
-
Targeted treatment of iron deficiency in prolonged critical illness: an opportunity to improve survival or not?Crit Care. 2021 Jun 1;25(1):188. doi: 10.1186/s13054-021-03590-w. Crit Care. 2021. PMID: 34074323 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

