Increased serum QUIN/KYNA is a reliable biomarker of post-stroke cognitive decline
- PMID: 33588894
- PMCID: PMC7885563
- DOI: 10.1186/s13024-020-00421-4
Increased serum QUIN/KYNA is a reliable biomarker of post-stroke cognitive decline
Abstract
Background: Strokes are becoming less severe due to increased numbers of intensive care units and improved treatments. As patients survive longer, post-stroke cognitive impairment (PSCI) has become a major health public issue. Diabetes has been identified as an independent predictive factor for PSCI. Here, we characterized a clinically relevant mouse model of PSCI, induced by permanent cerebral artery occlusion in diabetic mice, and investigated whether a reliable biomarker of PSCI may emerge from the kynurenine pathway which has been linked to inflammatory processes.
Methods: Cortical infarct was induced by permanent middle cerebral artery occlusion in male diabetic mice (streptozotocin IP). Six weeks later, cognitive assessment was performed using the Barnes maze, hippocampi long-term potentiation using microelectrodes array recordings, and neuronal death, white matter rarefaction and microglia/macrophages density assessed in both hemispheres using imunohistochemistry. Brain and serum metabolites of the kynurenin pathway were measured using HPLC and mass fragmentography. At last, these same metabolites were measured in the patient's serum, at the acute phase of stroke, to determine if they could predict PSCI 3 months later.
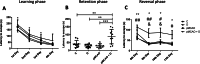
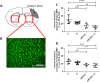
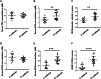
Results: We found long-term spatial memory was impaired in diabetic mice 6 weeks after stroke induction. Synaptic plasticity was completely suppressed in both hippocampi along with increased neuronal death, white matter rarefaction in both striatum, and increased microglial/macrophage density in the ipsilateral hemisphere. Brain and serum quinolinic acid concentrations and quinolinic acid over kynurenic acid ratios were significantly increased compared to control, diabetic and non-diabetic ischemic mice, where PSCI was absent. These putative serum biomarkers were strongly correlated with degradation of long-term memory, neuronal death, microglia/macrophage infiltration and white matter rarefaction. Moreover, we identified these same serum biomarkers as potential predictors of PSCI in a pilot study of stroke patients.
Conclusions: we have established and characterized a new model of PSCI, functionally and structurally, and we have shown that the QUIN/KYNA ratio could be used as a surrogate biomarker of PSCI, which may now be tested in large prospective studies of stroke patients.
Keywords: 3-dioxygenase; Post-stroke dementia • mouse • electrophysiology • tryptophane • cerebral ischemia • indolamine 2.
Conflict of interest statement
The authors declare that they have no competiting interests.
Figures







References
-
- Disease GB, Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
-
- Bowler JV, Hachinski V. Vascular cognitive impairment: a new approach to vascular dementia. Baillieres Clin Neurol. 1995;4:357–376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

