Long-term health-related quality of life in patients treated with subcutaneous C1-inhibitor replacement therapy for the prevention of hereditary angioedema attacks: findings from the COMPACT open-label extension study
- PMID: 33588897
- PMCID: PMC7885603
- DOI: 10.1186/s13023-020-01658-4
Long-term health-related quality of life in patients treated with subcutaneous C1-inhibitor replacement therapy for the prevention of hereditary angioedema attacks: findings from the COMPACT open-label extension study
Erratum in
-
Correction to: Long-term health-related quality of life in patients treated with subcutaneous C1-inhibitor replacement therapy for the prevention of hereditary angioedema attacks: findings from the COMPACT open-label extension study.Orphanet J Rare Dis. 2021 Jul 28;16(1):329. doi: 10.1186/s13023-021-01975-2. Orphanet J Rare Dis. 2021. PMID: 34321052 Free PMC article. No abstract available.
Abstract
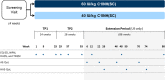
Background: Long-term prophylaxis with subcutaneous C1-inhibitor (C1-INH[SC]; HAEGARDA, CSL Behring) in patients with hereditary angioedema (HAE) due to C1-INH deficiency (C1-INH-HAE) was evaluated in an open-label extension follow-up study to the international, double-blind, placebo-controlled COMPACT study. The current analysis evaluated patient-reported health-related quality of life (HRQoL) data from 126 patients in the open-label extension study randomized to treatment with C1-INH(SC) 40 IU/kg (n = 63) or 60 IU/kg (n = 63) twice weekly for 52 weeks. HRQoL was evaluated at the beginning of the open-label study and at various time points using the European Quality of Life-5 Dimensions Questionnaire (EQ-5D), the Hospital Anxiety and Depression Scale (HADS), the Work Productivity and Activity Impairment Questionnaire (WPAI), and the Treatment Satisfaction Questionnaire for Medication. The disease-specific Angioedema Quality of Life Questionnaire (AE-QoL) and HAE quality of life questionnaire (HAE-QoL) instruments were administered in a subset of patients. Statistical significance was determined by change-from-baseline 95% confidence intervals (CIs) excluding zero. No adjustment for multiplicity was done.
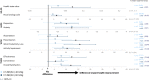
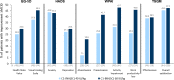
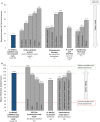
Results: Mean baseline EQ-5D scores (Health State Value, 0.90; Visual Analog Scale, 81.32) were slightly higher (better) than United States population norms (0.825, 80.0, respectively) and mean HADS anxiety (5.48) and depression (2.88) scores were within "normal" range (0-7). Yet, patients using C1-INH(SC) 60 IU/kg demonstrated significant improvement from baseline to end-of-study on the EQ-5D Health State Value (mean change [95% CI], 0.07 [0.01, 0.12] and Visual Analog Scale (7.45 [3.29, 11.62]). In the C1-INH(SC) 60 IU/kg group, there were significant improvements in the HADS anxiety scale (mean change [95% CI], - 1.23 [- 2.08, - 0.38]), HADS depression scale (- 0.95 [- 1.57, - 0.34]), and WPAI-assessed presenteeism (mean change [95% CI], - 23.33% [- 34.86, - 11.81]), work productivity loss (- 26.68% [- 39.92, - 13.44]), and activity impairment (- 16.14% [- 26.36, - 5.91]). Clinically important improvements were achieved in ≥ 25% of patients for all domains except WPAI-assessed absenteeism (which was very low at baseline). Mean AE-QoL total score by visit ranged from 13.39 to 17.89 (scale 0-100; lower scores = less impairment). Mean HAE-QoL global scores at each visit (115.7-122.3) were close to the maximum (best) possible score of 135.
Conclusions: Long-term C1-INH(SC) replacement therapy in patients with C1-INH-HAE leads to significant and sustained improvements in multiple measures of HRQoL. Trial registration A Study to Evaluate the Long-term Clinical Safety and Efficacy of Subcutaneously Administered C1-esterase Inhibitor in the Prevention of Hereditary Angioedema, NCT02316353. Registered December 12, 2014, https://clinicaltrials.gov/ct2/show/NCT02316353 .
Keywords: Anxiety; C1-inhibitor protein; Depression; HAEGARDA; Health-related quality of life; Hereditary angioedema; Patient-reported outcomes; Productivity; Subcutaneous.
Conflict of interest statement
W Lumry is a consultant for Adverum, Attune, BioCryst, CSL Behring, Kalvista, Pharming, and Takeda; a speaker for CSL Behring, Pharming, and Takeda; and has received research grants from BioCryst, CSL Behring, Pharming, Ionis, and Takeda. B Zuraw has served as a consultant for Adverum, Attune, BioCryst, CSL Behring, and Takeda. M Cicardi was a PI, consultant, and speaker for CSL Behring, Shire/Takeda, and Pharming; a consultant PI for BioCryst, Kalvista, Attune, and Pharvaris; and conducted research with Pharming and Shire/Takeda. T Craig is a consultant and speaker for CSL Behring, Shire/Takeda, Spark, and Pharming; has conducted research for CSL Behring, Shire/Takeda, Ionis, and BioCryst; and is on the Medical Advisory Board for the HAEA, Board of Directors for the AAAAI and the Board of Directors for ALA- Mid Atlantic. J Anderson is a PI, consultant, and speaker for CSL Behring, Shire/Takeda, and Pharming and a PI for BioCryst. A Banerji conducted research with Shire/Takeda, BioCryst and served as a consultant for Shire/Takeda, BioCryst, Pharming, CSL, and Kalvista. JA Bernstein is a PI, consultant, and speaker for CSL Behring, Shire/Takeda, and Pharming; a consultant and PI for BioCryst; and a consultant for Kalvista. T Caballero is a speaker for CSL Behring, Merck, Novartis, and Shire/Takeda; consultant for BioCryst, CSL Behring, Novartis, Octapharma, Pharming, and Shire/Takeda; she has been a PI in clinical trials/registries for BioCryst, CSL Behring, Novartis, Pharming, and Shire/Takeda; she has received educational funding from CSL Behring, Novartis, and Shire/Takeda; and she is a researcher from the IdiPAZ program for promoting research activities. H Farkas has received honoraria, speaker fees, and travel grants from CSL Behring, Shire/Takeda, Swedish Orphan Biovitrum, and Pharming and/or served as a consultant for these companies and has participated in clinical trials/registries for BioCryst, CSL Behring, Pharming, and Shire/Takeda. RG Gower has conducted research with Shire/Takeda and BioCryst; he has served as a consultant with Shire/Takeda and CSL Behring; is on the advisory board for Shire/Takeda, BioCryst, and Fresenius Kabi; and he has received speakers’ fees from Shire/Takeda. PK Keith has conducted research with, served as a consultant for, and has received speakers’ fees from Shire/Takeda and CSL Behring. DS Levy has served as a consultant, speaker, and has received research grants from CSL Behring; he has served as a consultant for BioCryst; he has served as a speaker for Takeda. HH Li has conducted research with and has served as a consultant/received speakers’ fees from Shire/Takeda, BioCryst, and CSL Behring; he has served as a consultant/received speakers’ fees from Pharming. M Magerl is a PI, consultant, and/or speaker for CSL Behring, BioCryst, Kalvista, Shire/Takeda, and Pharming. M Manning is a researcher/PI and speaker/consultant at CSL Behring, Shire/Takeda, BioCryst, and Pharming. M Riedl is a research investigator with CSL Behring, Takeda, BioCryst, and Ionis; he has served as a consultant for CSL Behring, Takeda, BioCryst, Pharming, Adverum, Attune, Kalvista, and Pharvaris; and he has received speakers’ fees from CSL Behring, Takeda, and Pharming. J-P Lawo, S Prusty, and T Machnig are/were employees of and stockholders at CSL Behring during study conduct. H Longhurst served as a consultant for, participated in research with or accepted educational funding support from Adverum, BioCryst, CSL Behring, GSK, Kalvista, Pfizer, Pharming, Pharvaris, and Shire/Takeda.
Figures
Similar articles
-
Health-Related Quality of Life with Subcutaneous C1-Inhibitor for Prevention of Attacks of Hereditary Angioedema.J Allergy Clin Immunol Pract. 2018 Sep-Oct;6(5):1733-1741.e3. doi: 10.1016/j.jaip.2017.12.039. Epub 2018 Jan 31. J Allergy Clin Immunol Pract. 2018. PMID: 29391286 Clinical Trial.
-
Long-Term Outcomes with Subcutaneous C1-Inhibitor Replacement Therapy for Prevention of Hereditary Angioedema Attacks.J Allergy Clin Immunol Pract. 2019 Jul-Aug;7(6):1793-1802.e2. doi: 10.1016/j.jaip.2019.01.054. Epub 2019 Feb 15. J Allergy Clin Immunol Pract. 2019. PMID: 30772477 Clinical Trial.
-
Preventive Treatment of Hereditary Angioedema: A Review of Phase III Clinical Trial Data for Subcutaneous C1 Inhibitor and Relevance for Patient Management.Clin Ther. 2021 Dec;43(12):2154-2166.e1. doi: 10.1016/j.clinthera.2021.10.008. Epub 2021 Dec 5. Clin Ther. 2021. PMID: 34879971 Review.
-
Subcutaneous C1 inhibitor for prevention of attacks of hereditary angioedema: additional outcomes and subgroup analysis of a placebo-controlled randomized study.Allergy Asthma Clin Immunol. 2019 Aug 28;15:49. doi: 10.1186/s13223-019-0362-1. eCollection 2019. Allergy Asthma Clin Immunol. 2019. PMID: 31485239 Free PMC article.
-
A Retrospective Analysis of Long-Term Prophylaxis with Berotralstat in Patients with Hereditary Angioedema and Acquired C1-Inhibitor Deficiency-Real-World Data.Clin Rev Allergy Immunol. 2023 Dec;65(3):354-364. doi: 10.1007/s12016-023-08972-2. Epub 2023 Nov 2. Clin Rev Allergy Immunol. 2023. PMID: 37914894 Free PMC article. Review.
Cited by
-
Hereditary angioedema prevalence and satisfaction with prophylaxis in South Australia.World Allergy Organ J. 2024 Jun 18;17(7):100918. doi: 10.1016/j.waojou.2024.100918. eCollection 2024 Jul. World Allergy Organ J. 2024. PMID: 39006039 Free PMC article.
-
Current challenges and future opportunities in patient-focused management of hereditary angioedema: A narrative review.Clin Transl Allergy. 2023 May;13(5):e12243. doi: 10.1002/clt2.12243. Clin Transl Allergy. 2023. PMID: 37227422 Free PMC article. Review.
-
The international WAO/EAACI guideline for the management of hereditary angioedema - The 2021 revision and update.World Allergy Organ J. 2022 Apr 7;15(3):100627. doi: 10.1016/j.waojou.2022.100627. eCollection 2022 Mar. World Allergy Organ J. 2022. PMID: 35497649 Free PMC article.
-
A narrative review of recent literature of the quality of life in hereditary angioedema patients.World Allergy Organ J. 2023 Mar 20;16(3):100758. doi: 10.1016/j.waojou.2023.100758. eCollection 2023 Mar. World Allergy Organ J. 2023. PMID: 36994443 Free PMC article. Review.
-
Real-world evidence of the effectiveness and utilization of subcutaneous C1INH long-term prophylaxis in patients with HAE in Spain and Germany.Front Immunol. 2025 May 14;16:1576235. doi: 10.3389/fimmu.2025.1576235. eCollection 2025. Front Immunol. 2025. PMID: 40438099 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

