MR CLEAN-NO IV: intravenous treatment followed by endovascular treatment versus direct endovascular treatment for acute ischemic stroke caused by a proximal intracranial occlusion-study protocol for a randomized clinical trial
- PMID: 33588908
- PMCID: PMC7885482
- DOI: 10.1186/s13063-021-05063-5
MR CLEAN-NO IV: intravenous treatment followed by endovascular treatment versus direct endovascular treatment for acute ischemic stroke caused by a proximal intracranial occlusion-study protocol for a randomized clinical trial
Abstract
Background: Endovascular treatment (EVT) has greatly improved the prognosis of acute ischemic stroke (AIS) patients with a proximal intracranial large vessel occlusion (LVO) of the anterior circulation. Currently, there is clinical equipoise concerning the added benefit of intravenous alteplase administration (IVT) prior to EVT. The aim of this study is to assess the efficacy and safety of omitting IVT before EVT in patients with AIS caused by an anterior circulation LVO.
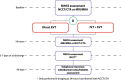
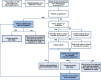
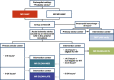
Methods: MR CLEAN-NO IV is a multicenter randomized open-label clinical trial with blinded outcome assessment (PROBE design). Patients ≥ 18 years of age with a pre-stroke mRS < 3 with an LVO confirmed on CT angiography/MR angiography eligible for both IVT and EVT are randomized to receive either IVT (0.9 mg/kg) followed by EVT, or direct EVT in a 1:1 ratio. The primary objective is to assess superiority of direct EVT. Secondarily, non-inferiority of direct EVT compared to IVT before EVT will be explored. The primary outcome is the score on the modified Rankin Scale at 90 days. Ordinal regression with adjustment for prognostic variables will be used to estimate treatment effect. Secondary outcomes include reperfusion graded with the eTICI scale after EVT and stroke severity (National Institutes of Health Stroke Scale) at 24 h. Safety outcomes include intracranial hemorrhages scored according to the Heidelberg criteria. A total of 540 patients will be included.
Discussion: IVT prior to EVT might facilitate early reperfusion before EVT or improved reperfusion rates during EVT. Conversely, among other potential adverse effects, the increased risk of bleeding could nullify the beneficial effects of IVT. MR CLEAN-NO IV will provide insight into whether IVT is still of added value in patients eligible for EVT.
Trial registration: www.isrctn.com : ISRCTN80619088 . Registered on 31 October 2017.
Keywords: Endovascular treatment; Intravenous alteplase; Ischemic stroke.
Conflict of interest statement
WZ reports that Maastricht University Medical Center received compensation from Stryker® and Cerenovus® for consultations by WvZ;
YR reports that he is a shareholder of Nico.lab
JMC reports that Amsterdam UMC received research support from Medtronic.
CM reports that Amsterdam UMC received research grants form CVON/Dutch Heart Foundation, European Commission, TWIN Foundation and Stryker®; he is a shareholder of Nico.lab.
DD and AvdL report that Erasmus MC received research grants from Dutch Heart Foundation, Dutch Brain Foundation, AngioCare BV, Covidien/EV3®, MEDAC Gmbh/LAMEPRO, Penumbra Inc., Stryker, and Top Medical/Concentric, The Netherlands Organization for Health Research and Development, Health Holland Top Sector Life Sciences & Health, Stryker, Thrombolytic Science, LLC, and Cerenovus.
All other authors declare that they have no competing interests.
Figures


References
-
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. - PubMed
-
- Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. - PubMed
-
- Saver JL, Goyal M, Bonafe A, Diener H-CC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95. - PubMed
-
- Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. - PubMed
-
- Jovin TG, Chamorro A, Cobo E, de Miquel MAA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

