Neutrophil dysfunction in cystic fibrosis
- PMID: 33589340
- PMCID: PMC8568301
- DOI: 10.1016/j.jcf.2021.01.012
Neutrophil dysfunction in cystic fibrosis
Abstract
Background: Excessive neutrophil inflammation is the hallmark of cystic fibrosis (CF) airway disease. Novel technologies for characterizing neutrophil dysfunction may provide insight into the nature of these abnormalities, revealing a greater mechanistic understanding and new avenues for CF therapies that target these mechanisms.
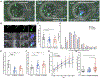
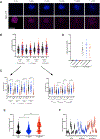
Methods: Blood was collected from individuals with CF in the outpatient clinic, CF individuals hospitalized for a pulmonary exacerbation, and non-CF controls. Using microfluidic assays and advanced imaging technologies, we characterized 1) spontaneous neutrophil migration using microfluidic motility mazes, 2) neutrophil migration to and phagocytosis of Staphylococcal aureus particles in a microfluidic arena, 3) neutrophil swarming on Candida albicans clusters, and 4) Pseudomonas aeruginosa-induced neutrophil transepithelial migration using micro-optical coherence technology (µOCT).
Results: Participants included 44 individuals: 16 Outpatient CF, 13 Hospitalized CF, and 15 Non-CF individuals. While no differences were seen with spontaneous migration, CF neutrophils migrated towards S. aureus particles more quickly than non-CF neutrophils (p < 0.05). CF neutrophils, especially Hospitalized CF neutrophils, generated significantly larger aggregates around S. aureus particles over time. Hospitalized CF neutrophils were more likely to have dysfunctional swarming (p < 0.01) and less efficient clearing of C. albicans (p < 0.0001). When comparing trans-epithelial migration towards Pseudomonas aeruginosa epithelial infection, Outpatient CF neutrophils displayed an increase in the magnitude of transmigration and adherence to the epithelium (p < 0.05).
Conclusions: Advanced technologies for characterizing CF neutrophil function reveal significantly altered migratory responses, cell-to-cell clustering, and microbe containment. Future investigations will probe mechanistic basis for abnormal responses in CF to identify potential avenues for novel anti-inflammatory therapeutics.
Keywords: Cystic fibrosis; Inflammation; Micro-fluidics; Micro-optical coherence tomography; Neutrophil.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing of Interest The authors have no conflicts of interest to disclose.
Figures


References
-
- Konstan MW, Walenga RW, Hilliard KA, Hilliard JB. Leukotriene B4 markedly elevated in the epithelial lining fluid of patients with cystic fibrosis. Am Rev Respir Dis 1993;148(4 Pt 1):896–901. - PubMed
-
- Ringholz FC, Buchanan PJ, Clarke DT, Millar RG, McDermott M, Linnane B, et al. Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. Eur Respir J 2014;44(2):394–404. - PubMed
-
- McElvaney OJ, Zaslona Z, Becker-Flegler K, Palsson-McDermott EM, Boland F, Gunaratnam C, et al. Specific Inhibition of the NLRP3 Inflammasome as an Antiinflammatory Strategy in Cystic Fibrosis. Am J Respir Crit Care Med 2019;200(11):1381–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

