TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis
- PMID: 33589622
- PMCID: PMC7884705
- DOI: 10.1038/s41419-021-03471-8
TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis
Abstract
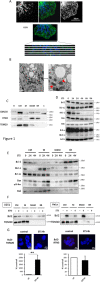
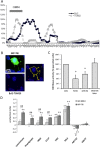
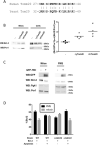
In this work, we have explored the subcellular localization of Bcl2, a major antiapoptotic protein. In U251 glioma cells, we found that Bcl2 is localized mainly in the ER and is translocated to MAM and mitochondria upon induction of apoptosis; this mitochondrial transfer was not restricted to the demonstrator cell line, even if cell-specific modulations exist. We found that the Bcl2/mitochondria interaction is controlled by TOM20, a protein that belongs to the protein import machinery of the mitochondrial outer membrane. The expression of a small domain of interaction of TOM20 with Bcl2 potentiates its anti-apoptotic properties, which suggests that the Bcl2-TOM20 interaction is proapoptotic. The role of MAM and TOM20 in Bcl2 apoptotic mitochondrial localization and function has been confirmed in a yeast model in which the ER-mitochondria encounter structure (ERMES) complex (required for MAM stability in yeast) has been disrupted. Bcl2-TOM20 interaction is thus an additional player in the control of apoptosis.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The pro-apoptotic BH3-only protein Bim interacts with components of the translocase of the outer mitochondrial membrane (TOM).PLoS One. 2015 Apr 15;10(4):e0123341. doi: 10.1371/journal.pone.0123341. eCollection 2015. PLoS One. 2015. PMID: 25875815 Free PMC article.
-
TOM70 Sustains Cell Bioenergetics by Promoting IP3R3-Mediated ER to Mitochondria Ca2+ Transfer.Curr Biol. 2018 Feb 5;28(3):369-382.e6. doi: 10.1016/j.cub.2017.12.047. Epub 2018 Jan 27. Curr Biol. 2018. PMID: 29395920
-
Interaction between the human mitochondrial import receptors Tom20 and Tom70 in vitro suggests a chaperone displacement mechanism.J Biol Chem. 2011 Sep 16;286(37):32208-19. doi: 10.1074/jbc.M111.280446. Epub 2011 Jul 19. J Biol Chem. 2011. PMID: 21771790 Free PMC article.
-
Role of Endoplasmic Reticulum-Mitochondria Communication in Type 2 Diabetes.Adv Exp Med Biol. 2017;997:171-186. doi: 10.1007/978-981-10-4567-7_13. Adv Exp Med Biol. 2017. PMID: 28815530 Review.
-
Alzheimer Disease.Adv Exp Med Biol. 2017;997:149-156. doi: 10.1007/978-981-10-4567-7_11. Adv Exp Med Biol. 2017. PMID: 28815528 Review.
Cited by
-
Contribution of Yeast Studies to the Understanding of BCL-2 Family Intracellular Trafficking.Int J Mol Sci. 2021 Apr 15;22(8):4086. doi: 10.3390/ijms22084086. Int J Mol Sci. 2021. PMID: 33920941 Free PMC article. Review.
-
Ratiometric measurement of MAM Ca2+ dynamics using a modified CalfluxVTN.Nat Commun. 2023 Jun 16;14(1):3586. doi: 10.1038/s41467-023-39343-2. Nat Commun. 2023. PMID: 37328454 Free PMC article.
-
Mitochondria-associated endoplasmic reticulum membrane (MAM): a dark horse for diabetic cardiomyopathy treatment.Cell Death Discov. 2024 Mar 20;10(1):148. doi: 10.1038/s41420-024-01918-3. Cell Death Discov. 2024. PMID: 38509100 Free PMC article. Review.
-
ER-mitochondria contact sites; a multifaceted factory for Ca2+ signaling and lipid transport.Front Cell Dev Biol. 2022 Aug 16;10:988014. doi: 10.3389/fcell.2022.988014. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36158205 Free PMC article. Review.
-
ER-SURF: Riding the Endoplasmic Reticulum Surface to Mitochondria.Int J Mol Sci. 2021 Sep 6;22(17):9655. doi: 10.3390/ijms22179655. Int J Mol Sci. 2021. PMID: 34502567 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

