Immunomodulation for optimal cardiac regeneration: insights from comparative analyses
- PMID: 33589632
- PMCID: PMC7884783
- DOI: 10.1038/s41536-021-00118-2
Immunomodulation for optimal cardiac regeneration: insights from comparative analyses
Abstract
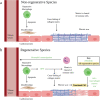
Despite decades of research, regeneration of the infarcted human heart remains an unmet ambition. A significant obstacle facing experimental regenerative therapies is the hostile immune response which arises following a myocardial infarction (MI). Upon cardiac damage, sterile inflammation commences via the release of pro-inflammatory meditators, leading to the migration of neutrophils, eosinophils and monocytes, as well as the activation of local vascular cells and fibroblasts. This response is amplified by components of the adaptive immune system. Moreover, the physical trauma of the infarction and immune-mediated tissue injury provides a supply of autoantigens, perpetuating a cycle of autoreactivity, which further contributes to adverse remodelling. A gradual shift towards an immune-resolving environment follows, culminating in the formation of a collagenous scar, which compromises cardiac function, ultimately driving the development of heart failure. Comparing the human heart with those of animal models that are capable of cardiac regeneration reveals key differences in the innate and adaptive immune responses to MI. By modulating key immune components to better resemble those of regenerative species, a cardiac environment may be established which would, either independently or via the synergistic application of emerging regenerative therapies, improve functional recovery post-MI.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Smart, N., Dubé, K. N. & Riley, P. R. Epicardial progenitor cells in cardiac regeneration and neovascularisation. Vascul. Pharmacol. 10.1016/j.vph.2012.08.001. (2013). - PubMed
-
- Bloom, D. E. et al. Methodological Appendix: the Global Economic Burden of Non-communicable Diseases. World Economic Forum (2011).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

