Molecular changes tracking through multiscale fluorescence microscopy differentiate Meningioma grades and non-tumoral brain tissues
- PMID: 33589651
- PMCID: PMC7884789
- DOI: 10.1038/s41598-020-78678-4
Molecular changes tracking through multiscale fluorescence microscopy differentiate Meningioma grades and non-tumoral brain tissues
Abstract
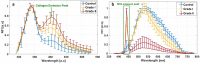
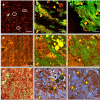
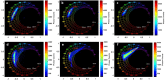
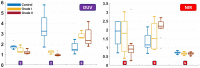
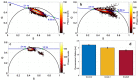
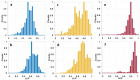
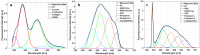
Meningioma is the most common primary intracranial extra-axial tumor. Total surgical removal is the standard therapeutic method to treat this type of brain tumors. However, the risk of recurrence depends on the tumor grade and the extent of the resection including the infiltrated dura mater and, if necessary, the infiltrated bone. Therefore, proper resection of all invasive tumor borders without touching eloquent areas is of primordial in order to decrease the risk of recurrence. Nowadays, none of the intraoperative used tools is able to provide a precise real-time histopathological information on the tumor surrounding areas to help the surgeon to achieve a gross total removal. To respond to this problem, our team is developing a multimodal two-photon fluorescence endomicroscope, compatible with the surgeon tool, to better delimitate tumor boundaries, relying on the endogenous fluorescence of brain tissues. In this context, we are building a tissue database in order to specify each brain tissue, whether healthy or tumoral, with its specific optical signature. In this study, we present a multimodal and multiscale optical measurements on non-tumoral control brain tissue obtained in epilepsy surgery patients and several meningioma grades. We investigated tissue auto-fluorescence to track the molecular changes associated with the tumor grade from deep ultra-violet (DUV) to near infrared (NIR) excitation. Micro-spectroscopy, fluorescence lifetime imaging, two-photon fluorescence imaging and Second Harmonic Generation (SHG) imaging were performed. Several optically derived parameters such as collagen crosslinks fluorescence in DUV, SHG emission in NIR and long lifetime intensity fraction of Nicotinamide Adenine Dinucleotide and Flavins were correlated to discriminate cancerous tissue from control one. While collagen response managed to discriminate meningioma grades from control samples with a 100% sensitivity and 90% specificity through a 3D discriminative algorithm.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Louis, D. N., Ohgaki, H., Wiestler, O. D. & Cavenee, W. K. WHO Classification of Tumours of the Central Nervous System (International Agency for Research on Cancer, 2016).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

