Palladin isoforms 3 and 4 regulate cancer-associated fibroblast pro-tumor functions in pancreatic ductal adenocarcinoma
- PMID: 33589694
- PMCID: PMC7884442
- DOI: 10.1038/s41598-021-82937-3
Palladin isoforms 3 and 4 regulate cancer-associated fibroblast pro-tumor functions in pancreatic ductal adenocarcinoma
Abstract
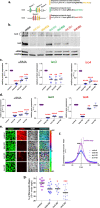
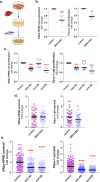
Pancreatic Ductal Adenocarcinoma (PDAC) has a five-year survival under 10%. Treatment is compromised due to a fibrotic-like stromal remodeling process, known as desmoplasia, which limits therapeutic perfusion, supports tumor progression, and establishes an immunosuppressive microenvironment. These processes are driven by cancer-associated fibroblasts (CAFs), functionally activated through transforming growth factor beta1 (TGFβ1). CAFs produce a topographically aligned extracellular matrix (ECM) that correlates with reduced overall survival. Paradoxically, ablation of CAF populations results in a more aggressive disease, suggesting CAFs can also restrain PDAC progression. Thus, unraveling the mechanism(s) underlying CAF functions could lead to therapies that reinstate the tumor-suppressive features of the pancreatic stroma. CAF activation involves the f-actin organizing protein palladin. CAFs express two palladin isoforms (iso3 and iso4) which are up-regulated in response to TGFβ1. However, the roles of iso3 and iso4 in CAF functions remain elusive. Using a CAF-derived ECM model, we uncovered that iso3/iso4 are required to sustain TGFβ1-dependent CAF activation, secrete immunosuppressive cytokines, and produce a pro-tumoral ECM. Findings demonstrate a novel role for CAF palladin and suggest that iso3/iso4 regulate both redundant and specific tumor-supportive desmoplastic functions. This study highlights the therapeutic potential of targeting CAFs to restore fibroblastic anti-tumor activity in the pancreatic microenvironment.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Recent advances in understanding cancer-associated fibroblasts in pancreatic cancer.Am J Physiol Cell Physiol. 2020 Aug 1;319(2):C233-C243. doi: 10.1152/ajpcell.00079.2020. Epub 2020 May 20. Am J Physiol Cell Physiol. 2020. PMID: 32432930 Free PMC article. Review.
-
Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma.J Pathol. 2019 May;248(1):51-65. doi: 10.1002/path.5224. Epub 2019 Feb 22. J Pathol. 2019. PMID: 30575030 Free PMC article.
-
Anti-metastatic potential of somatostatin analog SOM230: Indirect pharmacological targeting of pancreatic cancer-associated fibroblasts.Oncotarget. 2016 Jul 5;7(27):41584-41598. doi: 10.18632/oncotarget.9296. Oncotarget. 2016. PMID: 27177087 Free PMC article.
-
Bone marrow-derived macrophages converted into cancer-associated fibroblast-like cells promote pancreatic cancer progression.Cancer Lett. 2021 Aug 1;512:15-27. doi: 10.1016/j.canlet.2021.04.013. Epub 2021 May 5. Cancer Lett. 2021. PMID: 33961925
-
Heterogeneity and plasticity of cancer-associated fibroblasts in the pancreatic tumor microenvironment.Semin Cancer Biol. 2022 Jul;82:184-196. doi: 10.1016/j.semcancer.2021.03.006. Epub 2021 Mar 15. Semin Cancer Biol. 2022. PMID: 33737108 Review.
Cited by
-
The role of coagulome in the tumor immune microenvironment.Adv Drug Deliv Rev. 2023 Sep;200:115027. doi: 10.1016/j.addr.2023.115027. Epub 2023 Jul 28. Adv Drug Deliv Rev. 2023. PMID: 37517779 Free PMC article. Review.
-
Eribulin normalizes pancreatic cancer-associated fibroblasts by simulating selected features of TGFβ inhibition.BMC Cancer. 2022 Dec 2;22(1):1255. doi: 10.1186/s12885-022-10330-y. BMC Cancer. 2022. PMID: 36461015 Free PMC article.
-
The actin-binding protein palladin associates with the respiratory syncytial virus matrix protein.J Virol. 2024 Oct 22;98(10):e0143524. doi: 10.1128/jvi.01435-24. Epub 2024 Oct 3. J Virol. 2024. PMID: 39360826 Free PMC article.
-
Challenges and Opportunities Modeling the Dynamic Tumor Matrisome.BME Front. 2023 Jan 16;4:0006. doi: 10.34133/bmef.0006. eCollection 2023. BME Front. 2023. PMID: 37849664 Free PMC article. Review.
-
From precursor to cancer: decoding the intrinsic and extrinsic pathways of pancreatic intraepithelial neoplasia progression.Carcinogenesis. 2024 Nov 22;45(11):801-816. doi: 10.1093/carcin/bgae064. Carcinogenesis. 2024. PMID: 39514554 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

