Targeting the membrane-proximal C2-set domain of CD33 for improved CD33-directed immunotherapy
- PMID: 33589747
- PMCID: PMC8364569
- DOI: 10.1038/s41375-021-01160-1
Targeting the membrane-proximal C2-set domain of CD33 for improved CD33-directed immunotherapy
Abstract
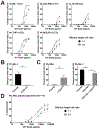
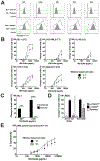
There is increasing interest in targeting CD33 in malignant and non-malignant disorders. In acute myeloid leukemia, longer survival with the CD33 antibody-drug conjugate gemtuzumab ozogamicin (GO) validates this strategy. Still, GO benefits only some patients, prompting efforts to develop more potent CD33-directed therapeutics. As one limitation, CD33 antibodies typically recognize the membrane-distal V-set domain. Using various artificial CD33 proteins, in which this domain was differentially positioned within the extracellular portion of the molecule, we tested whether targeting membrane-proximal epitopes enhances the effector functions of CD33 antibody-based therapeutics. Consistent with this idea, a CD33V-set/CD3 bispecific antibody (BsAb) and CD33V-set-directed chimeric antigen receptor (CAR)-modified T cells elicited substantially greater cytotoxicity against cells expressing a CD33 variant lacking the entire C2-set domain than cells expressing full-length CD33, whereas cytotoxic effects induced by GO were independent of the position of the V-set domain. We therefore raised murine and human antibodies against the C2-set domain of human CD33 and identified antibodies that bound CD33 regardless of the presence/absence of the V-set domain ("CD33PAN antibodies"). These antibodies internalized when bound to CD33 and, as CD33PAN/CD3 BsAb, had potent cytolytic effects against CD33+ cells. Together, our data provide the rationale for further development of CD33PAN antibody-based therapeutics.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited part of Springer Nature.
Figures


Similar articles
-
Expression and functional characterization of CD33 transcript variants in human acute myeloid leukemia.Oncotarget. 2016 Jul 12;7(28):43281-43294. doi: 10.18632/oncotarget.9674. Oncotarget. 2016. PMID: 27248327 Free PMC article.
-
T cell engaging bispecific antibodies targeting CD33 IgV and IgC domains for the treatment of acute myeloid leukemia.J Immunother Cancer. 2021 May;9(5):e002509. doi: 10.1136/jitc-2021-002509. J Immunother Cancer. 2021. PMID: 34035113 Free PMC article.
-
The past and future of CD33 as therapeutic target in acute myeloid leukemia.Blood Rev. 2014 Jul;28(4):143-53. doi: 10.1016/j.blre.2014.04.001. Epub 2014 Apr 21. Blood Rev. 2014. PMID: 24809231 Review.
-
CD33-directed immunotherapy with third-generation chimeric antigen receptor T cells and gemtuzumab ozogamicin in intact and CD33-edited acute myeloid leukemia and hematopoietic stem and progenitor cells.Int J Cancer. 2022 Apr 1;150(7):1141-1155. doi: 10.1002/ijc.33865. Epub 2021 Nov 23. Int J Cancer. 2022. PMID: 34766343
-
Investigational CD33-targeted therapeutics for acute myeloid leukemia.Expert Opin Investig Drugs. 2018 Apr;27(4):339-348. doi: 10.1080/13543784.2018.1452911. Epub 2018 Mar 15. Expert Opin Investig Drugs. 2018. PMID: 29534618 Review.
Cited by
-
Preclinical Characterization of the Anti-Leukemia Activity of the CD33/CD16a/NKG2D Immune-Modulating TriNKET® CC-96191.Cancers (Basel). 2024 Feb 22;16(5):877. doi: 10.3390/cancers16050877. Cancers (Basel). 2024. PMID: 38473239 Free PMC article.
-
Druggable genome-wide Mendelian randomization identifies therapeutic targets for metabolic dysfunction-associated steatotic liver disease.Lipids Health Dis. 2025 Mar 26;24(1):113. doi: 10.1186/s12944-025-02515-8. Lipids Health Dis. 2025. PMID: 40140823 Free PMC article.
-
Discovery and preclinical development of a SdAb-based CAR-T technology for targeting CD33 in AML.Mol Ther Oncol. 2025 Feb 11;33(1):200949. doi: 10.1016/j.omton.2025.200949. eCollection 2025 Mar 20. Mol Ther Oncol. 2025. PMID: 40084273 Free PMC article.
-
Optimizing Siglec-8-Directed Immunotherapy for Eosinophilic and Mast Cell Disorders.Cancers (Basel). 2024 Oct 14;16(20):3476. doi: 10.3390/cancers16203476. Cancers (Basel). 2024. PMID: 39456570 Free PMC article.
-
Targeting CD5 chimeric antigen receptor-engineered natural killer cells against T-cell malignancies.Exp Hematol Oncol. 2024 Oct 26;13(1):104. doi: 10.1186/s40164-024-00577-5. Exp Hematol Oncol. 2024. PMID: 39462383 Free PMC article.
References
-
- Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol 2020; 38: 365–395. - PubMed
-
- Grossbard ML, Press OW, Appelbaum FR, Bernstein ID, Nadler LM. Monoclonal antibody-based therapies of leukemia and lymphoma. Blood 1992; 80(4): 863–878. - PubMed
-
- Laszlo GS, Estey EH, Walter RB. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev 2014; 28(4): 143–153. - PubMed
-
- Walter RB. Expanding use of CD33-directed immunotherapy. Expert Opin Biol Ther 2020; 20(9): 955–958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

