Cytoplasmic DROSHA and non-canonical mechanisms of MiR-155 biogenesis in FLT3-ITD acute myeloid leukemia
- PMID: 33589748
- PMCID: PMC8973317
- DOI: 10.1038/s41375-021-01166-9
Cytoplasmic DROSHA and non-canonical mechanisms of MiR-155 biogenesis in FLT3-ITD acute myeloid leukemia
Erratum in
-
Correction: Cytoplasmic DROSHA and non-canonical mechanisms of MiR-155 biogenesis in FLT3-ITD acute myeloid leukemia.Leukemia. 2025 Jul;39(7):1795. doi: 10.1038/s41375-025-02637-z. Leukemia. 2025. PMID: 40325233 No abstract available.
Abstract
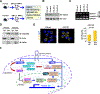
We report here on a novel pro-leukemogenic role of FMS-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD) that interferes with microRNAs (miRNAs) biogenesis in acute myeloid leukemia (AML) blasts. We showed that FLT3-ITD interferes with the canonical biogenesis of intron-hosted miRNAs such as miR-126, by phosphorylating SPRED1 protein and inhibiting the "gatekeeper" Exportin 5 (XPO5)/RAN-GTP complex that regulates the nucleus-to-cytoplasm transport of pre-miRNAs for completion of maturation into mature miRNAs. Of note, despite the blockage of "canonical" miRNA biogenesis, miR-155 remains upregulated in FLT3-ITD+ AML blasts, suggesting activation of alternative mechanisms of miRNA biogenesis that circumvent the XPO5/RAN-GTP blockage. MiR-155, a BIC-155 long noncoding (lnc) RNA-hosted oncogenic miRNA, has previously been implicated in FLT3-ITD+ AML blast hyperproliferation. We showed that FLT3-ITD upregulates miR-155 by inhibiting DDX3X, a protein implicated in the splicing of lncRNAs, via p-AKT. Inhibition of DDX3X increases unspliced BIC-155 that is then shuttled by NXF1 from the nucleus to the cytoplasm, where it is processed into mature miR-155 by cytoplasmic DROSHA, thereby bypassing the XPO5/RAN-GTP blockage via "non-canonical" mechanisms of miRNA biogenesis.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited part of Springer Nature.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

