Circadian rhythm as a therapeutic target
- PMID: 33589815
- PMCID: PMC8525418
- DOI: 10.1038/s41573-020-00109-w
Circadian rhythm as a therapeutic target
Abstract
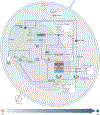
The circadian clock evolved in diverse organisms to integrate external environmental changes and internal physiology. The clock endows the host with temporal precision and robust adaptation to the surrounding environment. When circadian rhythms are perturbed or misaligned, as a result of jet lag, shiftwork or other lifestyle factors, adverse health consequences arise, and the risks of diseases such as cancer, cardiovascular diseases or metabolic disorders increase. Although the negative impact of circadian rhythm disruption is now well established, it remains underappreciated how to take advantage of biological timing, or correct it, for health benefits. In this Review, we provide an updated account of the circadian system and highlight several key disease areas with altered circadian signalling. We discuss environmental and lifestyle modifications of circadian rhythm and clock-based therapeutic strategies, including chronotherapy, in which dosing time is deliberately optimized for maximum therapeutic index, and pharmacological agents that target core clock components and proximal regulators. Promising progress in research, disease models and clinical applications should encourage a concerted effort towards a new era of circadian medicine.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



References
-
-
Reddy P et al. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38, 701–710 (1984).
This landmark article reports the discovery of the period (per) locus in Drosophila melanogaster by molecular mapping of chromosome aberrations.
-
-
-
Bargiello TA, Jackson FR & Young MW Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 312, 752–754 (1984).
This article demonstrates that introduction of a DNA fragment from a per+ fly encoding a 4.5-kb poly(A)+ RNA (later known as per) in a per0 (arrhythmic) fly can restore its circadian rhythmicity.
-
-
-
Hardin PE, Hall JC & Rosbash M Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343, 536–540 (1990).
This landmark article identifies a feedback loop through which the cyclic per RNA level is regulated by its own protein activity.
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

