Metabolic support of tumour-infiltrating regulatory T cells by lactic acid
- PMID: 33589820
- PMCID: PMC7990682
- DOI: 10.1038/s41586-020-03045-2
Metabolic support of tumour-infiltrating regulatory T cells by lactic acid
Abstract
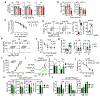
Regulatory T (Treg) cells, although vital for immune homeostasis, also represent a major barrier to anti-cancer immunity, as the tumour microenvironment (TME) promotes the recruitment, differentiation and activity of these cells1,2. Tumour cells show deregulated metabolism, leading to a metabolite-depleted, hypoxic and acidic TME3, which places infiltrating effector T cells in competition with the tumour for metabolites and impairs their function4-6. At the same time, Treg cells maintain a strong suppression of effector T cells within the TME7,8. As previous studies suggested that Treg cells possess a distinct metabolic profile from effector T cells9-11, we hypothesized that the altered metabolic landscape of the TME and increased activity of intratumoral Treg cells are linked. Here we show that Treg cells display broad heterogeneity in their metabolism of glucose within normal and transformed tissues, and can engage an alternative metabolic pathway to maintain suppressive function and proliferation. Glucose uptake correlates with poorer suppressive function and long-term instability, and high-glucose conditions impair the function and stability of Treg cells in vitro. Treg cells instead upregulate pathways involved in the metabolism of the glycolytic by-product lactic acid. Treg cells withstand high-lactate conditions, and treatment with lactate prevents the destabilizing effects of high-glucose conditions, generating intermediates necessary for proliferation. Deletion of MCT1-a lactate transporter-in Treg cells reveals that lactate uptake is dispensable for the function of peripheral Treg cells but required intratumorally, resulting in slowed tumour growth and an increased response to immunotherapy. Thus, Treg cells are metabolically flexible: they can use 'alternative' metabolites in the TME to maintain their suppressive identity. Further, our results suggest that tumours avoid destruction by not only depriving effector T cells of nutrients, but also metabolically supporting regulatory populations.
Conflict of interest statement
The authors declare no competing interests.
Figures





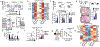

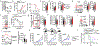
Comment in
-
Lactate-avid regulatory T cells: metabolic plasticity controls immunosuppression in tumour microenvironment.Signal Transduct Target Ther. 2021 Apr 30;6(1):171. doi: 10.1038/s41392-021-00598-0. Signal Transduct Target Ther. 2021. PMID: 33931598 Free PMC article. No abstract available.
-
Going sugar free: Treg cells avoid glucose to maintain functional fitness.Immunol Cell Biol. 2021 Jul;99(6):558-560. doi: 10.1111/imcb.12461. Epub 2021 May 20. Immunol Cell Biol. 2021. PMID: 34013589 No abstract available.
References
-
- Wang H, Franco F & Ho P-C Metabolic regulation of tregs in cancer: opportunities for immunotherapy. Trends Cancer 3, 583–592 (2017). - PubMed
-
- Sakaguchi S, Yamaguchi T, Nomura T & Ono M Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008). - PubMed
-
- Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DP2 AI136598/AI/NIAID NIH HHS/United States
- F31 AI149971/AI/NIAID NIH HHS/United States
- T32 CA082084/CA/NCI NIH HHS/United States
- F31 AI147638/AI/NIAID NIH HHS/United States
- S10 OD023402/OD/NIH HHS/United States
- S10 OD020007/OD/NIH HHS/United States
- P50 CA097190/CA/NCI NIH HHS/United States
- T32 AI089443/AI/NIAID NIH HHS/United States
- R01 DK089125/DK/NIDDK NIH HHS/United States
- R21 AI135367/AI/NIAID NIH HHS/United States
- P30 CA047904/CA/NCI NIH HHS/United States
- R01 NS099320/NS/NINDS NIH HHS/United States
- P50 CA121973/CA/NCI NIH HHS/United States
- F31 CA247129/CA/NCI NIH HHS/United States
- R01 CA203689/CA/NCI NIH HHS/United States
- P01 AI108545/AI/NIAID NIH HHS/United States
- R01 NS086818/NS/NINDS NIH HHS/United States
- F30 CA247034/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

