Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection
- PMID: 33589885
- PMCID: PMC7874960
- DOI: 10.1016/j.medj.2021.02.001
Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection
Abstract
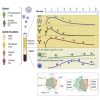
Background: Monitoring the adaptive immune responses during the natural course of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection provides useful information for the development of vaccination strategies against this virus and its emerging variants. We thus profiled the serum anti-SARS-CoV-2 antibody (Ab) levels and specific memory B and T cell responses in convalescent coronavirus disease 2019 (COVID-19) patients.
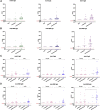
Methods: A total of 119 samples from 88 convalescent donors who experienced mild to critical disease were tested for the presence of elevated anti-spike and anti-receptor binding domain Ab levels over a period of 8 months. In addition, the levels of SARS-CoV-2 neutralizing Abs and specific memory B and T cell responses were tested in a subset of samples.
Findings: Anti-SARS-CoV-2 Abs were present in 85% of the samples collected within 4 weeks after the onset of symptoms in COVID-19 patients. Levels of specific immunoglobulin M (IgM)/IgA Abs declined after 1 month, while levels of specific IgG Abs and plasma neutralizing activities remained relatively stable up to 6 months after diagnosis. Anti-SARS-CoV-2 IgG Abs were still present, although at a significantly lower level, in 80% of the samples collected at 6-8 months after symptom onset. SARS-CoV-2-specific memory B and T cell responses developed with time and were persistent in all of the patients followed up for 6-8 months.
Conclusions: Our data suggest that protective adaptive immunity following natural infection of SARS-CoV-2 may persist for at least 6-8 months, regardless of disease severity. Development of medium- or long-term protective immunity through vaccination may thus be possible.
Funding: This project was supported by the European Union's Horizon 2020 research and innovation programme (ATAC, no. 101003650), the Italian Ministry of Health (Ricerca Finalizzata grant no. GR-2013-02358399), the Center for Innovative Medicine, and the Swedish Research Council. J.A. was supported by the SciLifeLab/KAW national COVID-19 research program project grant 2020.
Keywords: B cell; COVID-19; IgG; SARS-CoV-2; T cell; antibody; immunity; immunological memory; longevity of immune response; neutralizing antibody.
© 2021 The Author(s).
Conflict of interest statement
S.B.-A. is a member of the advisory board of Mabtech AB. The other authors declare no competing interests.
Figures




References
-
- Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165878. - PMC - PubMed
-
- Juno J.A., Tan H.-X., Lee W.S., Reynaldi A., Kelly H.G., Wragg K., Esterbauer R., Kent H.E., Batten C.J., Mordant F.L., et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020;26:1428–1434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

