Exploring the putative mechanism of allosteric modulations by mixed-action kappa/mu opioid receptor bitopic modulators
- PMID: 33590767
- PMCID: PMC8027703
- DOI: 10.4155/fmc-2020-0308
Exploring the putative mechanism of allosteric modulations by mixed-action kappa/mu opioid receptor bitopic modulators
Abstract
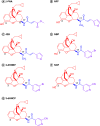
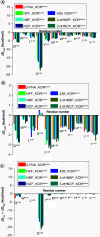
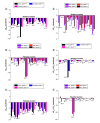
The modulation and selectivity mechanisms of seven mixed-action kappa opioid receptor (KOR)/mu opioid receptor (MOR) bitopic modulators were explored. Molecular modeling results indicated that the 'message' moiety of seven bitopic modulators shared the same binding mode with the orthosteric site of the KOR and MOR, whereas the 'address' moiety bound with different subdomains of the allosteric site of the KOR and MOR. The 'address' moiety of seven bitopic modulators bound to different subdomains of the allosteric site of the KOR and MOR may exhibit distinguishable allosteric modulations to the binding affinity and/or efficacy of the 'message' moiety. Moreover, the 3-hydroxy group on the phenolic moiety of the seven bitopic modulators induced selectivity to the KOR over the MOR.
Keywords: allosteric modulation mechanism; kappa opioid receptor (KOR)/mu opioid receptor (MOR); mixed-action KOR/MOR ligand; molecular dynamics simulations; psychostimulant abuse and addiction; selectivity.
Conflict of interest statement
This work was partially supported by the Virginia Commonwealth University Center for High-Performance Computing and NIH/National Institute on Drug Abuse grants R01DA024022, R01DA044855 and UG3DA050311 (Y Zhang) and R01 DA041359, R21 DA045274 and P30 DA013429 (L-Y Liu-Chen). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures



References
-
- Substance Abuse and Mental Health Services Administration. www.samhsa.gov/data/
-
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 51(1–2), 141–153 (1998). - PubMed
-
- Lott DC, Lott DC, Kim S-J et al. Serotonin transporter genotype and acute subjective response to amphetamine. Am. J. Addict. 15(5), 327–335 (2006). - PubMed
-
- Broadbear JH, Sumpter TL, Burke TF et al. Methocinnamox is a potent, long-lasting, and selective antagonist of morphine-mediated antinociception in the mouse: comparison with clocinnamox, β-funaltrexamine, and β-chlornaltrexamine. J. Pharmacol. Exp. Ther. 294(3), 933–940 (2000). - PubMed
-
- American Addiction Centers. https://americanaddictioncenters.org/stimulant-drugs
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
