Pra-C exerts analgesic effect through inhibiting microglial activation in anterior cingulate cortex in complete Freund's adjuvant-induced mouse model
- PMID: 33590786
- PMCID: PMC7894694
- DOI: 10.1177/1744806921990934
Pra-C exerts analgesic effect through inhibiting microglial activation in anterior cingulate cortex in complete Freund's adjuvant-induced mouse model
Abstract
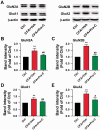
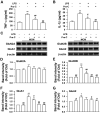
Chronic pain is highly prevalent worldwide and severely affects daily lives of patients and family members. Praeruptorin C (Pra-C) is a main active ingredient derived from Peucedanum praeruptorum Dunn, traditionally used as antibechic, anti-bronchitis and anti-hypertension drug. Here, we evaluated the effects of Pra-C in a chronic inflammatory pain mouse model induced by complete Freund's adjuvant (CFA) injection. Pra-C (3 mg/kg) treatment for just 3 days after CFA challenge relieved CFA-induced mechanical allodynia and hindpaw edema in mice. In the anterior cingulate cortex (ACC), Pra-C treatment inhibited microglia activation and reduced levels of proinflammatory cytokines, TNF-α and IL-1β, and suppressed upregulation of glutamate receptors caused by CFA injection. In addition, Pra-C attenuated neuronal hyperexcitability in ACC of CFA-injected mice. In vitro studies confirmed the analgesic effect of Pra-C was due to its inhibitory ability on microglial activation. In conclusion, Pra-C administration had a certain effect on relieving chronic pain by inhibiting microglial activation, attenuating proinflammatory cytokine releasing and regulating excitatory synaptic proteins in the ACC of the CFA-injected mice.
Keywords: Praeruptorin C; inflammatory pain; microglia; proinflammatory cytokine.
Conflict of interest statement
Figures



References
-
- Velly AM, Mohit S. Epidemiology of pain and relation to psychiatric disorders. Progr Neuro-psychopharmacol Biol Psychiatry 2018; 87: 159–167. - PubMed
-
- Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, Mao Y, Wang L, Xie W, Zhong H, Zhang N, Luo MH, Tao W, Wang H, Li J, Li J, Qiu BS, Zhou JN, Li X, Xu H, Wang K, Zhang X, Liu Y, Richter-Levin G, Xu L, Zhang Z. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci 2019; 22: 1649–1658. - PubMed
-
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain 2019; 160: 19–27. - PubMed
-
- Chen T, Taniguchi W, Chen QY, Tozaki-Saitoh H, Song Q, Liu RH, Koga K, Matsuda T, Kaito-Sugimura Y, Wang J, Li ZH, Lu YC, Inoue K, Tsuda M, Li YQ, Nakatsuka T, Zhuo M. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat Commun 2018; 9: 1886. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

