Upadacitinib improves patient-reported outcomes vs placebo or adalimumab in patients with rheumatoid arthritis: results from SELECT-COMPARE
- PMID: 33590829
- PMCID: PMC8645276
- DOI: 10.1093/rheumatology/keab158
Upadacitinib improves patient-reported outcomes vs placebo or adalimumab in patients with rheumatoid arthritis: results from SELECT-COMPARE
Abstract
Objective: To evaluate the impact of upadacitinib vs placebo and adalimumab treatment, on patient-reported outcomes (PROs) in SELECT-COMPARE in an active RA population with inadequate responses to MTX (MTX-IR).
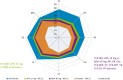
Methods: PROs in patients receiving upadacitinib (15 mg QD), placebo, or adalimumab (40 mg EOW) while on background MTX were evaluated over 48 weeks. PROs included Patient Global Assessment of Disease Activity (PtGA) and pain by visual analogue scale (VAS), the HAQ Disability Index (HAQ-DI), the 36-Item Short Form Survey (SF-36), morning (AM) stiffness duration and severity, the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), and work instability. Least squares mean (LSM) changes and proportions of patients reporting improvements ≥ minimal clinically important differences (MCIDs) and scores ≥ normative values were evaluated.
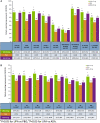
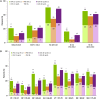
Results: Upadacitinib and adalimumab resulted in greater LSM changes from baseline vs placebo across all PROs (P < 0.05) at week 12, and pain and AM stiffness severity (P < 0.05) at week 2. More upadacitinib- vs placebo-treated (P < 0.05) and similar percentages of upadacitinib- vs adalimumab-treated patients reported improvements ≥ MCID across all PROs at week 12. Upadacitinib vs adalimumab resulted in greater LSM changes from baseline in PtGA, pain, HAQ-DI, stiffness severity, FACIT-F, and the SF-36 Physical Component Summary (PCS) (all P < 0.05) at week 12. More upadacitinib- vs adalimumab-treated patients reported scores ≥ normative values in HAQ-DI and SF-36 PCS (P < 0.05) at week 12. More upadacitinib- vs adalimumab-treated patients maintained clinically meaningful improvements in PtGA, pain, HAQ-DI, FACIT-F, and AM stiffness through 48 weeks.
Conclusion: In MTX-IR patients with RA, treatment with upadacitinib resulted in statistically significant and clinically meaningful improvements in PROs equivalent to or greater than with adalimumab.
Trial registration: ClinicalTrials.gov, http://clinicaltrials.gov, NCT02629159.
Keywords: DMARDs; inflammation; outcome measures; quality of life; rheumatoid arthritis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
References
-
- Smolen JS, Aletaha D, McInnes IB.. Rheumatoid arthritis. Lancet 2016;388:2023–38. - PubMed
-
- Hewlett S, Cockshott Z, Byron M. et al. Patients’ perceptions of fatigue in rheumatoid arthritis: overwhelming, uncontrollable, ignored. Arthritis Rheum 2005;53:697–702. - PubMed
-
- Pollard LC, Choy EH, Gonzalez J. et al. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford) 2006;45:885–9. - PubMed
-
- Westhoff G, Buttgereit F, Gromnica-Ihle E. et al. Morning stiffness and its influence on early retirement in patients with recent onset rheumatoid arthritis. Rheumatology (Oxford) 2008;47:980–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

