A critical residue in the α1M2-M3 linker regulating mammalian GABAA receptor pore gating by diazepam
- PMID: 33591271
- PMCID: PMC7899671
- DOI: 10.7554/eLife.64400
A critical residue in the α1M2-M3 linker regulating mammalian GABAA receptor pore gating by diazepam
Abstract
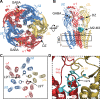
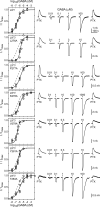
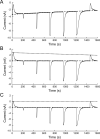
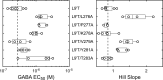
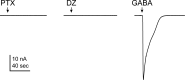
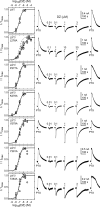
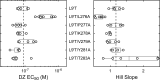
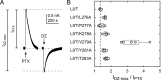
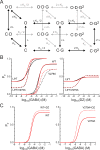
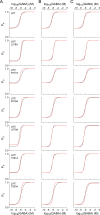
Benzodiazepines (BZDs) are a class of widely prescribed psychotropic drugs that modulate activity of GABAA receptors (GABAARs), neurotransmitter-gated ion channels critical for synaptic transmission. However, the physical basis of this modulation is poorly understood. We explore the role of an important gating domain, the α1M2-M3 linker, in linkage between the BZD site and pore gate. To probe energetics of this coupling without complication from bound agonist, we use a gain of function mutant (α1L9'Tβ2γ2L) directly activated by BZDs. We identify a specific residue whose mutation (α1V279A) more than doubles the energetic contribution of the BZD positive modulator diazepam (DZ) to pore opening and also enhances DZ potentiation of GABA-evoked currents in a wild-type background. In contrast, other linker mutations have little effect on DZ efficiency, but generally impair unliganded pore opening. Our observations reveal an important residue regulating BZD-pore linkage, thereby shedding new light on the molecular mechanism of these drugs.
Keywords: GABAA receptor; M2-M3 linker; allosteric modulator; benzodiazepine; diazepam; molecular biophysics; structural biology; xenopus.
© 2021, Nors et al.
Conflict of interest statement
JN, SG, MG No competing interests declared
Figures





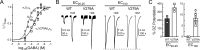
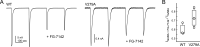
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

