A spinoparabrachial circuit defined by Tacr1 expression drives pain
- PMID: 33591273
- PMCID: PMC7993995
- DOI: 10.7554/eLife.61135
A spinoparabrachial circuit defined by Tacr1 expression drives pain
Abstract
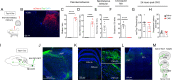
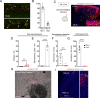
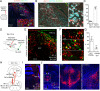
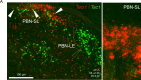
Painful stimuli evoke a mixture of sensations, negative emotions and behaviors. These myriad effects are thought to be produced by parallel ascending circuits working in combination. Here, we describe a pathway from spinal cord to brain for ongoing pain. Activation of a subset of spinal neurons expressing Tacr1 evokes a full repertoire of somatotopically directed pain-related behaviors in the absence of noxious input. Tacr1 projection neurons (expressing NKR1) target a tiny cluster of neurons in the superior lateral parabrachial nucleus (PBN-SL). We show that these neurons, which also express Tacr1 (PBN-SLTacr1), are responsive to sustained but not acute noxious stimuli. Activation of PBN-SLTacr1 neurons alone did not trigger pain responses but instead served to dramatically heighten nocifensive behaviors and suppress itch. Remarkably, mice with silenced PBN-SLTacr1 neurons ignored long-lasting noxious stimuli. Together, these data reveal new details about this spinoparabrachial pathway and its key role in the sensation of ongoing pain.
Keywords: brainstem; mouse; neuroscience; pain; parabrachial nucleus; somatosensation; spinal cord.
Conflict of interest statement
AB, AS, JT, MS, AL, AC No competing interests declared
Figures






References
-
- Abdus-Saboor I, Fried NT, Lay M, Burdge J, Swanson K, Fischer R, Jones J, Dong P, Cai W, Guo X, Tao YX, Bethea J, Ma M, Dong X, Ding L, Luo W. Development of a mouse pain scale using Sub-second behavioral mapping and statistical modeling. Cell Reports. 2019;28:1623–1634. doi: 10.1016/j.celrep.2019.07.017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

