Long-term outcome of (neo)adjuvant zoledronic acid therapy in locally advanced breast cancer
- PMID: 33591469
- PMCID: PMC8068643
- DOI: 10.1007/s10549-021-06100-2
Long-term outcome of (neo)adjuvant zoledronic acid therapy in locally advanced breast cancer
Abstract
Purpose: The role of zoledronic acid (ZOL), a bone-targeted bisphosphonate, in the treatment of patients with breast cancer remains an active area of study. Here, we report the long-term outcomes of a randomized placebo-controlled phase II clinical trial in which ZOL treatment was added to neoadjuvant chemotherapy in women with locally advanced breast cancer.
Methods: 120 women with clinical stage II-III (≥ T2 and/or ≥ N1) newly diagnosed breast cancer were randomized to receive either 4 mg intravenous ZOL every 3 weeks for 1 year (17 total doses) beginning with the first dose of neoadjuvant chemotherapy, or chemotherapy alone. Clinical endpoints included time to recurrence (TTR), time to bone recurrence (TTBR), time to non-bone recurrence (TTNBR), breast cancer survival (BCS) and overall survival (OS).
Results: With a median follow-up interval of 14.4 years, there were no significant differences in any of the clinical endpoints studied between the control and ZOL groups in the overall study population. However, ER+/HER2- patients younger than age 45 who were treated with ZOL had significantly worse TTR and TTNBR with a trend towards worse TTBR, BCS and OS (TTR: P = 0.024, HR 6.05 [1.26-29.1]; TTNBR: P = 0.026, HR 6.94 [1.26-38.1]; TTBR: P = 0.054, HR 6.01 [0.97-37.1]; BCS: P = 0.138, HR 4.43 [0.62-31.7]; OS: P = 0.138, HR 4.43 [0.62-31.7]). These differences were not seen in older ER+/HER2- patients or triple-negative patients of any age.
Conclusion: Addition of ZOL to neoadjuvant therapy did not significantly affect clinical outcomes in the overall study population but was associated with increased extra-skeletal recurrence and a trend towards worse survival in ER+/HER2- patients younger than age 45. These findings suggest caution when using zoledronic acid in young, premenopausal women with locally advanced breast cancer and warrant further investigation. Clinical Trial Registration Number NCT00242203, Date of Registration: 10/17/2005.
Keywords: Bisphosphonate; Breast cancer; Neoadjuvant; Premenopausal; Zoledronic acid.
Conflict of interest statement
Conflicts of Interest
The authors declare that they have no conflict of interest.
Figures




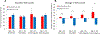
References
-
- Reddy SM, Barcenas CH, Sinha AK, Hsu L, Moulder SL, Tripathy D, Hortobagyi GN, Valero V (2018) Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease free at 5 years and relationship with low hormone receptor positivity. British Journal of Cancer 118:17–23. 10.1038/bjc.2017.379 - DOI - PMC - PubMed
-
- Braun S, Janni W, Schlimok G, Gebauer G, Oruzio D, Kundt G, Wong GYC, Pantel K (2005) A Pooled Analysis of Bone Marrow Micrometastasis in Breast Cancer. The New England Journal of Medicine 10 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

