A real-world data of Immune checkpoint inhibitors in solid tumors from India
- PMID: 33591635
- PMCID: PMC7940210
- DOI: 10.1002/cam4.3617
A real-world data of Immune checkpoint inhibitors in solid tumors from India
Abstract
Background: Checkpoint inhibitors (Nivolumab and Pembrolizumab) are approved for multiple indications in solid tumors. However access to these therapies is limited in low and middle income countries. Hence we performed an audit to identify accessibility, adverse event rates, compliance, progression free survival and overall survival in solid tumors.
Methods: This was a single center retrospective analysis of prospective data base of patients with non-melanoma solid tumors who were treated with immunotherapy from August 2015 to November 2018. Adverse events during immunotherapy were documented and graded using CTCAE (Common terminology criteria for adverse events), v. 4.02. The response rates to immunotherapy, toxicities and the time to onset and resolution of toxicities were also evaluated as secondary endpoints.
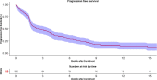
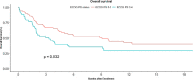
Results: Out of 9610 patients, only 155 patients (1.61%) could receive immunotherapy. The most common malignancies included metastatic non-small cell lung cancer, metastatic renal cell carcinoma, metastatic urothelial carcinoma and relapsed/recurrent head and neck squamous cell carcinoma. Median overall survival in patients who received immunotherapy in non-melanoma solid malignancies was 5.37 months (95% CI, 3.73-9.73). Poor performance status at baseline was the only adverse prognostic factor. The median progression free survival was 2.57 months (95% CI, 1.73-3.83). Immunotherapy was well tolerated with most common side effects being fatigue 14.8% and anorexia 5.8%. The cumulative incidence of immune related adverse events like hepatitis, pneumonitis, colitis and nephritis was less than 10%.
Conclusion: Real-world data in Indian setting confirms the benefit of immunotherapy in patients with advanced non-melanoma solid tumors.
Keywords: CPI; Immunotherapy; India; Nivolumab; Pembrolizumab; checkpoint inhibitors; hepatitis; irAE; pneumonitis.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures






Similar articles
-
Clinical outcomes of immune checkpoint inhibitors for patients with recurrent or metastatic head and neck cancer: real-world data in Korea.BMC Cancer. 2020 Aug 5;20(1):727. doi: 10.1186/s12885-020-07214-4. BMC Cancer. 2020. PMID: 32758163 Free PMC article.
-
Cancer Site and Adverse Events Induced by Immune Checkpoint Inhibitors: A Retrospective Analysis of Real-life Experience at a Single Institution.Anticancer Res. 2019 Feb;39(2):781-790. doi: 10.21873/anticanres.13175. Anticancer Res. 2019. PMID: 30711957 Free PMC article.
-
Real-world data on patients with metastatic non-small-cell lung cancer treated with checkpoint inhibitors in an Italian Teaching Hospital in 2015-2018.J Oncol Pharm Pract. 2021 Jun;27(4):877-886. doi: 10.1177/1078155220941586. Epub 2020 Jul 19. J Oncol Pharm Pract. 2021. PMID: 32686615
-
Anti-PD-1/L1-associated immune-related adverse events as harbinger of favorable clinical outcome: systematic review and meta-analysis.Clin Transl Oncol. 2021 Jan;23(1):100-109. doi: 10.1007/s12094-020-02397-5. Epub 2020 Jun 3. Clin Transl Oncol. 2021. PMID: 32495269
-
The effects of checkpoint inhibition on head and neck squamous cell carcinoma: A systematic review.Oral Oncol. 2019 Mar;90:67-73. doi: 10.1016/j.oraloncology.2019.01.018. Epub 2019 Feb 5. Oral Oncol. 2019. PMID: 30846179
Cited by
-
Durvalumab Plus Gemcitabine and Cisplatin Versus Gemcitabine and Cisplatin in Biliary Tract Cancer: a Real-World Retrospective, Multicenter Study.Target Oncol. 2024 May;19(3):359-370. doi: 10.1007/s11523-024-01060-1. Epub 2024 May 1. Target Oncol. 2024. PMID: 38691295
-
Precision Medicine and Clinical Trials in Advanced and Metastatic Oral Cancer.J Maxillofac Oral Surg. 2024 Aug;23(4):772-782. doi: 10.1007/s12663-024-02254-w. Epub 2024 Jun 17. J Maxillofac Oral Surg. 2024. PMID: 39118916 Free PMC article. Review.
-
Extended duration of treatment using reduced-frequency dosing of anti-PD-1 therapy in patients with advanced melanoma and Merkel cell carcinoma.Cancer Immunol Immunother. 2023 Nov;72(11):3839-3850. doi: 10.1007/s00262-023-03539-8. Epub 2023 Sep 21. Cancer Immunol Immunother. 2023. PMID: 37733060 Free PMC article.
-
Addressing the affordability gap of novel cancer treatments in developing countries.PLOS Digit Health. 2024 May 1;3(5):e0000488. doi: 10.1371/journal.pdig.0000488. eCollection 2024 May. PLOS Digit Health. 2024. PMID: 38691523 Free PMC article. No abstract available.
-
Real-world experience of second-line axitinib in metastatic renal cell carcinoma: analysis of the Swedish population.Future Oncol. 2024;20(20):1385-1392. doi: 10.1080/14796694.2024.2351352. Epub 2024 Jul 26. Future Oncol. 2024. PMID: 39057291 Free PMC article.
References
-
- Pham T, Roth S, Kong J, et al. An update on immunotherapy for solid tumors: a review. Ann Surg Oncol. 2018;25(11):3404‐3412. - PubMed
-
- Clarke JM, George DJ, Lisi S, Salama AKS. Immune checkpoint blockade: the new frontier in cancer treatment. Target Oncol. 2018;13(1):1‐20. - PubMed
-
- Rausch MP, Hastings KT. Immune checkpoint inhibitors in the treatment of melanoma: from basic science to clinical application. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy [Internet]. Brisbane (AU): Codon Publications; 2017. Available from: http://www.ncbi.nlm.nih.gov/books/NBK481851/ - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

