CRISPR/Cas9-mediated CysLT1R deletion reverses synaptic failure, amyloidosis and cognitive impairment in APP/PS1 mice
- PMID: 33591941
- PMCID: PMC7993729
- DOI: 10.18632/aging.202501
CRISPR/Cas9-mediated CysLT1R deletion reverses synaptic failure, amyloidosis and cognitive impairment in APP/PS1 mice
Abstract
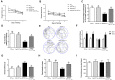
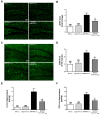
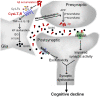
As a major pathological hallmark of Alzheimer's disease (AD), amyloid-β (Aβ) is regarded as a causative factor for cognitive impairment. Extensive studies have found Aβ induces a series of pathophysiological responses, finally leading to memory loss in AD. Our previous results demonstrated that cysteinyl leukotrienes receptor 1 (CysLT1R) antagonists improved exogenous Aβ-induced memory impairment. But the role of CysLT1R in AD and its underlying mechanisms still remain elusive. In this study, we investigated CysLT1R levels in AD patients and APP/PS1 mice. We also generated APP/PS1-CysLT1R-/- mice by clustered regulatory interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9)-mediated CysLT1R deletion in APP/PS1 mice and studied the effect of CysLT1R knockout on amyloidogenesis, synapse structure and plasticity, cognition, neuroinflammation, and kynurenine pathway. These attributes were also studied after lentivirus-mediated knockdown of CysLT1R gene in APP/PS1 mice. We found that CysLT1R knockout or knockdown could conserve synaptic structure and plasticity, and improve cognition in APP/PS1 mice. These effects were associated with concurrent decreases in amyloid processing, reduced neuroinflammation and suppression of the kynurenine pathway. Our study demonstrates that CysLT1R deficiency can mediate several beneficial effects against AD pathogenesis, and genetic/pharmacological ablation of this protein could be a potential therapeutic option for AD.
Keywords: Alzheimer’s disease; amyloidogenesis; cognition; cysteinyl leukotrienes receptor 1; synaptic plasticity.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

