Computational prediction of drug response in short QT syndrome type 1 based on measurements of compound effect in stem cell-derived cardiomyocytes
- PMID: 33591962
- PMCID: PMC7909705
- DOI: 10.1371/journal.pcbi.1008089
Computational prediction of drug response in short QT syndrome type 1 based on measurements of compound effect in stem cell-derived cardiomyocytes
Abstract
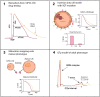
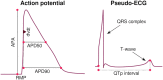
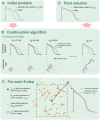
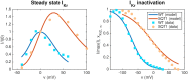
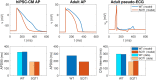
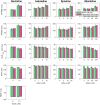
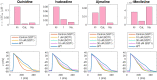
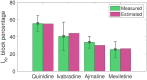
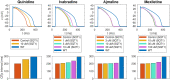
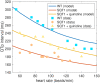
Short QT (SQT) syndrome is a genetic cardiac disorder characterized by an abbreviated QT interval of the patient's electrocardiogram. The syndrome is associated with increased risk of arrhythmia and sudden cardiac death and can arise from a number of ion channel mutations. Cardiomyocytes derived from induced pluripotent stem cells generated from SQT patients (SQT hiPSC-CMs) provide promising platforms for testing pharmacological treatments directly in human cardiac cells exhibiting mutations specific for the syndrome. However, a difficulty is posed by the relative immaturity of hiPSC-CMs, with the possibility that drug effects observed in SQT hiPSC-CMs could be very different from the corresponding drug effect in vivo. In this paper, we apply a multistep computational procedure for translating measured drug effects from these cells to human QT response. This process first detects drug effects on individual ion channels based on measurements of SQT hiPSC-CMs and then uses these results to estimate the drug effects on ventricular action potentials and QT intervals of adult SQT patients. We find that the procedure is able to identify IC50 values in line with measured values for the four drugs quinidine, ivabradine, ajmaline and mexiletine. In addition, the predicted effect of quinidine on the adult QT interval is in good agreement with measured effects of quinidine for adult patients. Consequently, the computational procedure appears to be a useful tool for helping predicting adult drug responses from pure in vitro measurements of patient derived cell lines.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: KJ, SW and AT have financial relationships with Organos Inc, and the company may benefit from commercialization of the results of this research.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

