An open label, block randomized, community study of the safety and efficacy of co-administered ivermectin, diethylcarbamazine plus albendazole vs. diethylcarbamazine plus albendazole for lymphatic filariasis in India
- PMID: 33591979
- PMCID: PMC7909694
- DOI: 10.1371/journal.pntd.0009069
An open label, block randomized, community study of the safety and efficacy of co-administered ivermectin, diethylcarbamazine plus albendazole vs. diethylcarbamazine plus albendazole for lymphatic filariasis in India
Abstract
Background: Better drug regimens for mass drug administration (MDA) could accelerate the Global Programme to Eliminate Lymphatic Filariasis (LF). This community study was designed to compare the safety and efficacy of MDA with IDA (ivermectin, diethylcarbamazine and albendazole) or DA (diethylcarbamazine and albendazole) in India.
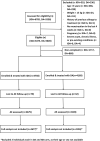
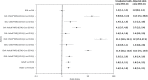
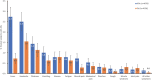
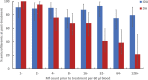
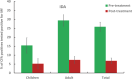
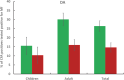
Methodology/principal findings: This two-armed, open-labelled, block randomised, community study was conducted in LF endemic villages in Yadgir district, Karnataka, India. Consenting participants ≥5 years of age were tested for circulating filarial antigenemia (CFA) and microfilaremia (Mf) before treatment with a single oral dose of IDA or DA. Adverse events (AEs) were monitored actively for two days and passively for five more days. Persons with positive CFA or Mf tests at baseline were retested 12-months post-treatment to assess treatment efficacy. Baseline CFA and Mf-rates were 26.4% and 6.9% in IDA and 24.5% and 6.4% in DA villages respectively. 4758 and 4160 participants received IDA and DA. Most AEs were mild after both treatments; fewer than 0.1% of participants experienced AEs with severity > grade 1. No serious AEs were observed. Fever, headache and dizziness were the most common AEs. AE rates were slightly higher after IDA than DA (8.3% vs. 6.4%, P<0.01). AEs were more frequent in females and Mf-positives after either treatment, but significantly more frequent after IDA (40.5% vs 20.2%, P < 0.001). IDA was more effective for clearing Mf than DA (84% vs. 61.8%, P < 0.001). Geometric mean Mf counts per 60μl in retested Mf-positives decreased by 96.4% from 11.8 after IDA and by 90.0% from 9.5 after DA. Neither treatment was effective for clearing CFA.
Conclusions/significance: IDA had an acceptable safety profile and was more effective for clearing Mf than DA. With adequate compliance and medical support to manage AEs, IDA has the potential to accelerate LF elimination in India.
Trial registration: Clinical Trial Registry of India (CTRI No/2016/10/007399).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Safety and efficacy of mass drug administration with a single-dose triple-drug regimen of albendazole + diethylcarbamazine + ivermectin for lymphatic filariasis in Papua New Guinea: An open-label, cluster-randomised trial.PLoS Negl Trop Dis. 2022 Feb 9;16(2):e0010096. doi: 10.1371/journal.pntd.0010096. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35139070 Free PMC article. Clinical Trial.
-
Safety and efficacy of co-administered diethylcarbamazine, albendazole and ivermectin during mass drug administration for lymphatic filariasis in Haiti: Results from a two-armed, open-label, cluster-randomized, community study.PLoS Negl Trop Dis. 2020 Jun 8;14(6):e0008298. doi: 10.1371/journal.pntd.0008298. eCollection 2020 Jun. PLoS Negl Trop Dis. 2020. PMID: 32511226 Free PMC article. Clinical Trial.
-
Safety and effectiveness of triple-drug therapy with ivermectin, diethylcarbamazine, and albendazole in reducing lymphatic filariasis prevalence and clearing circulating filarial antigens in Mombasa, Kenya.Infect Dis Poverty. 2025 Feb 24;14(1):11. doi: 10.1186/s40249-025-01282-z. Infect Dis Poverty. 2025. PMID: 39994719 Free PMC article.
-
Adverse events following single dose treatment of lymphatic filariasis: Observations from a review of the literature.PLoS Negl Trop Dis. 2018 May 16;12(5):e0006454. doi: 10.1371/journal.pntd.0006454. eCollection 2018 May. PLoS Negl Trop Dis. 2018. PMID: 29768412 Free PMC article. Review.
-
Antifilarial treatment strategies: a systematic review and network meta-analysis.BMC Infect Dis. 2025 May 16;25(1):712. doi: 10.1186/s12879-025-11105-z. BMC Infect Dis. 2025. PMID: 40380307 Free PMC article. Review.
Cited by
-
Safety and efficacy of mass drug administration with a single-dose triple-drug regimen of albendazole + diethylcarbamazine + ivermectin for lymphatic filariasis in Papua New Guinea: An open-label, cluster-randomised trial.PLoS Negl Trop Dis. 2022 Feb 9;16(2):e0010096. doi: 10.1371/journal.pntd.0010096. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35139070 Free PMC article. Clinical Trial.
-
Albendazole and Mebendazole as Anti-Parasitic and Anti-Cancer Agents: an Update.Korean J Parasitol. 2021 Jun;59(3):189-225. doi: 10.3347/kjp.2021.59.3.189. Epub 2021 Jun 21. Korean J Parasitol. 2021. PMID: 34218593 Free PMC article. Review.
-
Implementation of Mass Drug Administration for Lymphatic Filariasis in Madagascar: The Progress, Effectiveness and Financial Savings of Integrating into an Existing Polio Campaign.Res Rep Trop Med. 2024 Dec 27;15:123-147. doi: 10.2147/RRTM.S487163. eCollection 2024. Res Rep Trop Med. 2024. PMID: 39741701 Free PMC article.
-
Evaluation of Microfilaremic Individuals after Mass Drug Treatment with Ivermectin, Diethylcarbamazine, and Albendazole for Lymphatic Filariasis in Papua New Guinea.Am J Trop Med Hyg. 2025 Mar 25;112(6):1235-1239. doi: 10.4269/ajtmh.24-0382. Print 2025 Jun 4. Am J Trop Med Hyg. 2025. PMID: 40132219 Free PMC article.
-
Global insights can accelerate India's journey towards the elimination of lymphatic filariasis as a public health problem.BMJ Glob Health. 2025 Jul 10;10(7):e018851. doi: 10.1136/bmjgh-2025-018851. BMJ Glob Health. 2025. PMID: 40639855 Free PMC article. Review.
References
-
- World Health Organization. Global programme to eliminate lymphatic filariasis: progress report, 2017. Wkly Epidemiol Rec. 2018;91(44):589–604. . - PubMed
-
- Roy N. Elimination of lymphatic filariasis–India: Updates and way forward. Manipal Journal of Medical Sciences. 2018;3(2):1–3.
-
- World Health Organization. WHO Guideline: Alternative mass drug administration regimens to eliminate lymphatic filariasis. 2017;(Contract No.: WHO/HTM/NTD/PCT/2017.07.). - PubMed
-
- World Health Organization. Global programme to eliminate lymphatic filariasis: progress report, 2018. Wkly Epidemiol Rec. 2019;94(41):457–72. Epub 11 OCTOBER 2019. . - PubMed
-
- Thomsen EK, Sanuku N, Baea M, Satofan S, Maki E, Lombore B, et al.. Efficacy, Safety, and Pharmacokinetics of Coadministered Diethylcarbamazine, Albendazole, and Ivermectin for Treatment of Bancroftian Filariasis. Clin Infect Dis. 2016;62(3):334–41. Epub 2015/10/22. 10.1093/cid/civ882 . - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

