A Neuroskeletal Atlas: Spatial Mapping and Contextualization of Axon Subtypes Innervating the Long Bones of C3H and B6 Mice
- PMID: 33592122
- PMCID: PMC8252627
- DOI: 10.1002/jbmr.4273
A Neuroskeletal Atlas: Spatial Mapping and Contextualization of Axon Subtypes Innervating the Long Bones of C3H and B6 Mice
Abstract
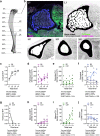
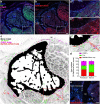
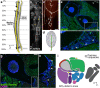
Nerves in bone play well-established roles in pain and vasoregulation and have been associated with progression of skeletal disorders, including osteoporosis, fracture, arthritis, and tumor metastasis. However, isolation of the region-specific mechanisms underlying these relationships is limited by our lack of quantitative methods for neuroskeletal analysis and precise maps of skeletal innervation. To overcome these limitations, we developed an optimized workflow for imaging and quantitative analysis of axons in and around the bone, including validation of Baf53b-Cre in concert with R26R-tdTomato (Ai9) as a robust pan-neuronal reporter system for use in musculoskeletal tissues. In addition, we created comprehensive maps of sympathetic adrenergic and sensory peptidergic axons within and around the full length of the femur and tibia in two strains of mice (B6 and C3H). In the periosteum, these maps were related to the surrounding musculature, including entheses and myotendinous attachments to bone. Three distinct patterns of periosteal innervation (termed type I, II, III) were defined at sites that are important for bone pain, bone repair, and skeletal homeostasis. For the first time, our results establish a gradient of bone marrow axon density that increases from proximal to distal along the length of the tibia and define key regions of interest for neuroskeletal studies. Lastly, this information was related to major nerve branches and local maps of specialized mechanoreceptors. This detailed mapping and contextualization of the axonal subtypes innervating the skeleton is intended to serve as a guide during the design, implementation, and interpretation of future neuroskeletal studies and was compiled as a resource for the field as part of the NIH SPARC consortium. © 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR)..
Keywords: BONE-BRAIN-NERVOUS SYSTEM INTERACTIONS; BONE-FAT INTERACTIONS; BONE-MUSCLE INTERACTIONS.
© 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

