Cathepsin K maintains the compartment of bone marrow T lymphocytes in vivo
- PMID: 33592138
- PMCID: PMC8127559
- DOI: 10.1002/iid3.412
Cathepsin K maintains the compartment of bone marrow T lymphocytes in vivo
Abstract
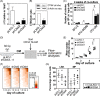
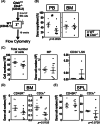
In this study, we investigated the influence of the loss of cathepsin K (Ctsk) gene on the hematopoietic system in vitro and in vivo. We found that cultures with lineage- SCA1+ KIT+ (LSK) cells on Ctsk deficient stromal cells display reduced colony formation and proliferation, with increased differentiation, giving rise to repopulating cells with reduced ability to repopulate the donor LSKs and T cell compartments in the bone marrow (BM). Subsequent in vivo experiments showed impairment of lymphocyte numbers, but, gross effects on early hematopoiesis or myelopoiesis were not found. Most consistently in in vivo experimental settings, we found a significant reduction of (donor) T cell numbers in the BM. Lymphocyte deregulation is also found in transplantation experiments, which revealed that Ctsk is required for optimal regeneration of small populations of T cells, particularly in the BM, but also of thymic B cells. Interestingly, cell nonautonomous Ctsk regulates both B and T cell numbers, but T cell numbers in the BM require an additional autonomous Ctsk-dependent process. Thus, we show that Ctsk is required for the maintenance of hematopoietic stem cells in vitro, but in vivo, Ctsk deficiency most strongly affects lymphocyte homeostasis, particularly of T cells in the BM.
Keywords: CTSK; cathepsin; cathepsin K; hematopoietic stem cells; lymphopoiesis; marrow; microenvironment; niche; stem cells.
© 2021 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

