Neoadjuvant Chemotherapy-Guided Bladder-Sparing Treatment for Muscle-Invasive Bladder Cancer: Results of a Pilot Phase II Study
- PMID: 33592141
- PMCID: PMC8524034
- DOI: 10.4143/crt.2020.1356
Neoadjuvant Chemotherapy-Guided Bladder-Sparing Treatment for Muscle-Invasive Bladder Cancer: Results of a Pilot Phase II Study
Abstract
Purpose: Reduced quality of life after cystectomy has made bladder preservation a popular research topic for muscle-invasive bladder cancer (MIBC). Previous research has indicated significant tumor downstaging after neoadjuvant chemotherapy (NAC). However, maximal transurethral resection of bladder tumor (TURBT) was performed before NAC to define the pathology, impacting the real evaluation of NAC. This research aimed to assess real NAC efficacy without interference from TURBT and apply combined modality therapies guided by NAC efficacy.
Materials and methods: Patients with cT2-4aN0M0 MIBC were confirmed by cystoscopic biopsy and imaging. NAC efficacy was assessed by imaging, urine cytology, and cystoscopy with multidisciplinary team discussion. Definite responders (≤ T1) underwent TURBT plus concurrent chemoradiotherapy. Incomplete responders underwent radical cystectomy or partial cystectomy if feasible. The primary endpoint was the bladder preservation rate.
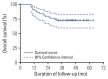
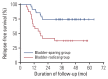
Results: Fifty-nine patients were enrolled, and the median age was 63 years. Patients with cT3-4 accounted for 75%. The median number of NAC cycles was three. Definite responders were 52.5%. The complete response (CR) was 10.2%, and 59.3% of patients received bladder-sparing treatments. With a median follow-up of 44.6 months, the 3-year overall survival (OS) was 72.8%. Three-year OS and relapse-free survival were 88.4% and 60.0% in the bladder-sparing group but only 74.3% and 37.5% in the cystectomy group. The evaluations of preserved bladder function were satisfactory.
Conclusion: After stratifying MIBC patients by NAC efficacy, definite responders achieved a satisfactory bladder-sparing rate, prognosis, and bladder function. The CR rate reflected the real NAC efficacy for MIBC. This therapy is worth verifying through multicenter research.
Keywords: Chemoradiotherapy; Combined modality therapy; Muscle-invasive bladder cancer; Neoadjuvant therapy; Organ sparing treatments.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures


References
-
- Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71:96–108. - PubMed
-
- Flaig TW. NCCN guidelines updates: management of muscle-invasive bladder cancer. J Natl Compr Canc Netw. 2019;17:591–3. - PubMed
-
- International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party; European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico Group, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–7. - PMC - PubMed
-
- Parekh DJ, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018;391:2525–36. - PubMed
-
- Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Choueiri TK, et al. Comparative analysis of outcomes and costs following open radical cystectomy versus robot-assisted laparoscopic radical cystectomy: results from the US Nationwide Inpatient Sample. Eur Urol. 2012;61:1239–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

