Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis
- PMID: 33592174
- PMCID: PMC7935772
- DOI: 10.1016/j.cell.2021.01.022
Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis
Abstract
T cells are critical effectors of cancer immunotherapies, but little is known about their gene expression programs in diffuse gliomas. Here, we leverage single-cell RNA sequencing (RNA-seq) to chart the gene expression and clonal landscape of tumor-infiltrating T cells across 31 patients with isocitrate dehydrogenase (IDH) wild-type glioblastoma and IDH mutant glioma. We identify potential effectors of anti-tumor immunity in subsets of T cells that co-express cytotoxic programs and several natural killer (NK) cell genes. Analysis of clonally expanded tumor-infiltrating T cells further identifies the NK gene KLRB1 (encoding CD161) as a candidate inhibitory receptor. Accordingly, genetic inactivation of KLRB1 or antibody-mediated CD161 blockade enhances T cell-mediated killing of glioma cells in vitro and their anti-tumor function in vivo. KLRB1 and its associated transcriptional program are also expressed by substantial T cell populations in other human cancers. Our work provides an atlas of T cells in gliomas and highlights CD161 and other NK cell receptors as immunotherapy targets.
Keywords: CD161; IDH-mutant gliomas; T cells; glioblastoma; single-cell RNA-seq.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests N.D.M., O.A., I.T., A.R., M.L.S., and K.W.W. are co-inventors of a patent application on the CLEC2D-CD161 pathway for the treatment of cancer. K.W.W., M.L.S., and A.R. are co-founders of Immunitas Therapeutics. K.W.W., M.L.S., and I.T. are advisory board members of Immunitas Therapeutics. K.W.W. serves on the scientific advisory board of TCR2 Therapeutics, T-Scan Therapeutics, SQZ Biotech, and Nextechinvest and received sponsored research funding from Bristol-Myers Squibb and Novartis. A.R. is a founder and equity holder of Celsius Therapeutics, an equity holder in Immunitas Therapeutics, and until August 31, 2020, was an SAB member of Syros Pharmaceuticals, Neogene Therapeutics, Asimov, and Thermo Fisher Scientific. From August 1, 2020, A.R. is an employee of Genentech. N.D.M. serves as a scientific advisor to Immunitas Therapeutics. A.M. is cofounder, member of the Boards of Directors, and member of Scientific Advisory Boards of Spotlight Therapeutics and Arsenal Biosciences. A.M. has served as an advisor to Juno Therapeutics, was a member of the scientific advisory board at PACT Pharma, and an advisor to Trizell. A.M. has received an honorarium from Merck and a consulting fee from AlphaSights and is an investor in and informal advisor to Offline Ventures. A.M. owns stock in Arsenal Biosciences, Spotlight Therapeutics, and PACT Pharma. The Marson lab has received research support from Juno Therapeutics, Epinomics, Sanofi, GlaxoSmithKline, Gilead, and Anthem. E.A.C. is currently an advisor to Advantagene Inc., Alcyone Biosciences, Insightec, Inc., Sigilon Therepeutics, and DNAtrix Inc. and has equity interest in DNAtrix; A.M. has advised Oncorus, Merck, Tocagen, Ziopharm, Stemgen, NanoTx., Ziopharm Oncology, Cerebral Therapeutics, Genenta, Merck, Janssen, Karcinolysis, and Shanaghai Biotech and has received research support from Advantagene, NewLink Genetics, and Amgen. A.M. is a named inventor on patents related to oncolytic HSV1. D.A.R. has received research support from Acerta Phamaceuticals, Agenus, Celldex, EMD Serono, Incyte, Inovio, Midatech, Omniox, and Tragara, and he has served as paid consultant for Abbvie, Advantagene, Agenus, Amgen, Bayer, Bristol-Myers Squibb, Celldex, DelMar, EMD Serono, Genentech/Roche, Inovio, Merck, Merck KGaA, Monteris, Novocure, Oncorus, Oxigene, Regeneron, Stemline, and Taiho Oncology, Inc. P.K.B., outside the scope of this work, has consulted for Angiochem, Genentech-Roche, Lilly, Tesaro, ElevateBio, Pfizer (Array), SK Life Sciences, and Dantari and is supported by the Breast Cancer Research Foundation, Damon Runyon Cancer Research Foundation, Ben and Catherine Ivy Foundation, and the National Cancer Institute (5R01CA244975-02, 5R21CA220253-02, and 5R01CA227156-03), BMS, Lilly, and honoraria from Merck, Genentech-Roche, and Lilly. D.P.C. has consulted for Lilly and Boston Pharmaceuticals and has received honoraria and travel reimbursement from Merck. O.R.-R. is an employee of Genentech since October 2020. O.R.-R. is a co-inventor on patent applications filed by the Broad Institute for inventions relating to work in single-cell genomics, such as in PCT/US2018/060860 and US provisional application 62/745,259. E.A.C. is currently an advisor to Advantagene Inc., Alcyone Biosciences, Insightec Inc., DNAtrix, Immunomic Therapeutics, Sangamo Therapeutics, and Seneca Therapeutics, has equity interest in DNAtrix, Immunomic Therapeutics, and Seneca Therapeutics, and has also advised Oncorus, Merck, Tocagen, Ziopharm, Stemgen, NanoTx., Ziopharm Oncology, Cerebral Therapeutics, Genenta. Merck, Janssen, Karcinolysis, and Shanghai Biotech. E.A.C. has received research support from NIH, US Department of Defense, American Brain Tumor Association, National Brain Tumor Society, Alliance for Cancer Gene Therapy, Neurosurgical Research Education Foundation, Advantagene, NewLink Genetics, and Amgen and also is a named inventor on patents related to oncolytic HSV1 and noncoding RNAs. X.S.L. is a cofounder, board member, and consultant of GV20 Oncotherapy and its subsidiaries, SAB of 3DMedCare, a consultant for Genentech, a stockholder of Bristol Myers Squibb (BMY), Thermo Fisher Scientific (TMO), Walgreens Boots Alliance (WBA), Abbott Laboratories (ABT), AbbVie Inc. (ABBV), and Johnson & Johnson (JNJ), and receives research funding from Takeda and Sanofi.
Figures
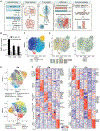


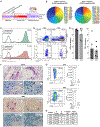

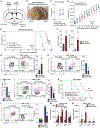

Comment in
-
T cell atlas reveals route to glioma immunotherapy.Nat Rev Drug Discov. 2021 Apr;20(4):261. doi: 10.1038/d41573-021-00038-2. Nat Rev Drug Discov. 2021. PMID: 33654224 No abstract available.
-
Brain tumor T cells inhibited by their natural KLR(B1) instinct.Sci Immunol. 2021 Apr 2;6(58):eabi7302. doi: 10.1126/sciimmunol.abi7302. Sci Immunol. 2021. PMID: 33811061
References
-
- Aldemir H, Prod'homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, Bihl F, and Braud VM (2005). Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol 175, 7791–7795. - PubMed
-
- Blondel VD, Guillaume J-L, Lambiotte R, and Lefebvre E (2008). Fast unfolding of communities in large networks. Journal of Statistical Mechanics: Theory and Experiment.
-
- Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, Alansary D, Sonner JK, Green E, Deumelandt K, et al. (2018). Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med 24, 1192–1203. - PubMed
-
- Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, Ritchie DS, Colonna M, Andrews DM, and Smyth MJ (2014). The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol 15, 431–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA227156/CA/NCI NIH HHS/United States
- R01 CA227821/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- K12 CA090354/CA/NCI NIH HHS/United States
- R21 CA220253/CA/NCI NIH HHS/United States
- R33 CA202820/CA/NCI NIH HHS/United States
- P50 CA165962/CA/NCI NIH HHS/United States
- R01 CA234018/CA/NCI NIH HHS/United States
- U19 CA264504/CA/NCI NIH HHS/United States
- U24 CA180922/CA/NCI NIH HHS/United States
- R01 CA238039/CA/NCI NIH HHS/United States
- P01 CA236749/CA/NCI NIH HHS/United States
- R01 CA251599/CA/NCI NIH HHS/United States
- R37 CA245523/CA/NCI NIH HHS/United States
- R01 CA244975/CA/NCI NIH HHS/United States
- L30 CA231679/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

