Optimal symptom combinations to aid COVID-19 case identification: Analysis from a community-based, prospective, observational cohort
- PMID: 33592254
- PMCID: PMC7881291
- DOI: 10.1016/j.jinf.2021.02.015
Optimal symptom combinations to aid COVID-19 case identification: Analysis from a community-based, prospective, observational cohort
Abstract
Objectives: Diagnostic work-up following any COVID-19 associated symptom will lead to extensive testing, potentially overwhelming laboratory capacity whilst primarily yielding negative results. We aimed to identify optimal symptom combinations to capture most cases using fewer tests with implications for COVID-19 vaccine developers across different resource settings and public health.
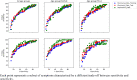
Methods: UK and US users of the COVID-19 Symptom Study app who reported new-onset symptoms and an RT-PCR test within seven days of symptom onset were included. Sensitivity, specificity, and number of RT-PCR tests needed to identify one case (test per case [TPC]) were calculated for different symptom combinations. A multi-objective evolutionary algorithm was applied to generate combinations with optimal trade-offs between sensitivity and specificity.
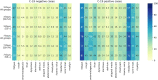
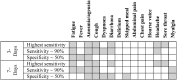
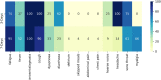
Findings: UK and US cohorts included 122,305 (1,202 positives) and 3,162 (79 positive) individuals. Within three days of symptom onset, the COVID-19 specific symptom combination (cough, dyspnoea, fever, anosmia/ageusia) identified 69% of cases requiring 47 TPC. The combination with highest sensitivity (fatigue, anosmia/ageusia, cough, diarrhoea, headache, sore throat) identified 96% cases requiring 96 TPC.
Interpretation: We confirmed the significance of COVID-19 specific symptoms for triggering RT-PCR and identified additional symptom combinations with optimal trade-offs between sensitivity and specificity that maximize case capture given different resource settings.
Keywords: COVID-19; Community-based cohort; Optimal symptom combinations; SARS-CoV-2; Vaccine trials.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest Potential conflicts of interest. JW, RD, JCP, and AM are employees of Zoe Global Ltd. ATC reports grants from Massachusetts Consortium on Pathogen Readiness during the conduct of the study, personal fees from Pfizer Inc., and grants and personal fees from Bayer Pharma; CEPI (authors AC, JG, JPC, AEL) funds clinical trials of COVID-19 vaccines. All other authors declare no competing interests.
Figures
Update of
-
Optimal symptom combinations to aid COVID-19 case identification: analysis from a community-based, prospective, observational cohort.medRxiv [Preprint]. 2021 Feb 8:2020.11.23.20237313. doi: 10.1101/2020.11.23.20237313. medRxiv. 2021. Update in: J Infect. 2021 Mar;82(3):384-390. doi: 10.1016/j.jinf.2021.02.015. PMID: 33269364 Free PMC article. Updated. Preprint.
References
-
- Corey L., Mascola J.R., Fauci A.S., Collins F.S. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368(6494):948–950. - PubMed
-
- Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine: https://investors.modernatx.com/node/10421/pdf [Accessed 9th December 2020].
-
- AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19: https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.... [Accessed 9th December 2020].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

