Sensory neurons derived from diabetic rats exhibit deficits in functional glycolysis and ATP that are ameliorated by IGF-1
- PMID: 33592336
- PMCID: PMC7940986
- DOI: 10.1016/j.molmet.2021.101191
Sensory neurons derived from diabetic rats exhibit deficits in functional glycolysis and ATP that are ameliorated by IGF-1
Abstract
Objective: The distal dying-back of the longest nerve fibres is a hallmark of diabetic neuropathy, and impaired provision of energy in the form of adenosine triphosphate (ATP) may contribute to this neurodegenerative process. We hypothesised that energy supplementation via glycolysis and/or mitochondrial oxidative phosphorylation is compromised in cultured dorsal root ganglion (DRG) sensory neurons from diabetic rodents, thus contributing to axonal degeneration. Functional analysis of glycolysis and mitochondrial respiration and real-time measurement of ATP levels in live cells were our specific means to test this hypothesis.
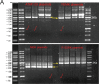
Methods: DRG neuron cultures from age-matched control or streptozotocin (STZ)-induced type 1 diabetic rats were used for in vitro studies. Three plasmids containing ATP biosensors of varying affinities were transfected into neurons to study endogenous ATP levels in real time. The Seahorse XF analyser was used for glycolysis and mitochondrial respiration measurements.
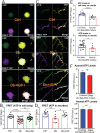
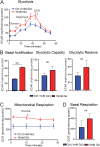
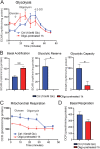
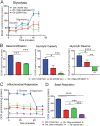
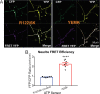
Results: Fluorescence resonance energy transfer (FRET) efficiency (YFP/CFP ratio) of the ATP biosensors AT1.03 (low affinity) and AT1.03YEMK (medium affinity) were significantly higher than that measured using the ATP-insensitive construct AT1.03R122/6K in both cell bodies and neurites of DRG neurons (p < 0.0001). The ATP level was homogenous along the axons but higher in cell bodies in cultured DRG neurons from both control and diabetic rats. Treatment with oligomycin (an ATP synthase inhibitor in mitochondria) decreased the ATP levels in cultured DRG neurons. Likewise, blockade of glycolysis using 2-deoxy-d-glucose (2-DG: a glucose analogue) reduced ATP levels (p < 0.001). Cultured DRG neurons derived from diabetic rats showed a diminishment of ATP levels (p < 0.01), glycolytic capacity, glycolytic reserve and non-glycolytic acidification. Application of insulin-like growth factor-1 (IGF-1) significantly elevated all the above parameters in DRG neurons from diabetic rats. Oligomycin pre-treatment of DRG neurons, to block oxidative phosphorylation, depleted the glycolytic reserve and lowered basal respiration in sensory neurons derived from control and diabetic rats. Depletion was much higher in sensory neurons from diabetic rats compared to control rats. In addition, an acute increase in glucose concentration, in the presence or absence of oligomycin, elevated parameters of glycolysis by 1.5- to 2-fold while having no impact on mitochondrial respiration.
Conclusion: We provide the first functional evidence for decreased glycolytic capacity in DRG neurons derived from type 1 diabetic rats. IGF-1 protected against the loss of ATP supplies in DRG cell bodies and axons in neurons derived from diabetic rats by augmenting various parameters of glycolysis and mitochondrial respiration.
Keywords: ATP biosensors; Axon regeneration; Bioenergetics; Diabetic neuropathy; Mitochondrial respiration; Neurite outgrowth.
Copyright © 2021 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures




References
-
- Chen H., Chan D.C. Critical dependence of neurons on mitochondrial dynamics. Current Opinion in Cell Biology. 2006;18(4):453–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

