Loss of CASK Accelerates Heart Failure Development
- PMID: 33593074
- PMCID: PMC8049978
- DOI: 10.1161/CIRCRESAHA.120.318170
Loss of CASK Accelerates Heart Failure Development
Abstract
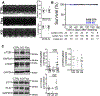
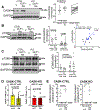
[Figure: see text].
Keywords: calmodulin; heart failure; myocardium; neurons; sarcoplasmic reticulum.
Conflict of interest statement
DISCLOSURES
The authors declare that no conflict of interest exists.
Figures





References
-
- MAIER LS, BERS DM. CALCIUM, CALMODULIN, AND CALCIUM-CALMODULIN KINASE II: HEARTBEAT TO HEARTBEAT AND BEYOND. J MOL CELL CARDIOL 2002;34:919–939. - PubMed
-
- WAGNER S, RUFF HM, WEBER SL, BELLMANN S, SOWA T, SCHULTE T, ANDERSON ME, GRANDI E, BERS DM, BACKS J, BELARDINELLI L, MAIER LS. REACTIVE OXYGEN SPECIES–ACTIVATED CA/CALMODULIN KINASE IIΔ IS REQUIRED FOR LATE INA AUGMENTATION LEADING TO CELLULAR NA AND CA OVERLOAD. CIRC RES 2011;108:555–565. - PMC - PubMed
-
- NEEF S, DYBKOVA N, SOSSALLA S, ORT KR, FLUSCHNIK N, NEUMANN K, SEIPELT R, SCHÖNDUBE FA, HASENFUSS G, MAIER LS. CAMKII-DEPENDENT DIASTOLIC SR CA2+ LEAK AND ELEVATED DIASTOLIC CA2+ LEVELS IN RIGHT ATRIAL MYOCARDIUM OF PATIENTS WITH ATRIAL FIBRILLATION. CIRC RES 2010;106:1134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

