Cell Surface Glycoprotein CD24 Marks Bone Marrow-Derived Human Mesenchymal Stem/Stromal Cells with Reduced Proliferative and Differentiation Capacity In Vitro
- PMID: 33593128
- PMCID: PMC7984936
- DOI: 10.1089/scd.2021.0027
Cell Surface Glycoprotein CD24 Marks Bone Marrow-Derived Human Mesenchymal Stem/Stromal Cells with Reduced Proliferative and Differentiation Capacity In Vitro
Abstract
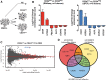
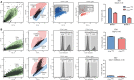
Bone marrow-derived mesenchymal stem/stromal cells (BMSCs) are fundamental to bone regenerative therapies, tissue engineering, and postmenopausal osteoporosis. Donor variation among patients, cell heterogeneity, and unpredictable capacity for differentiation reduce effectiveness of BMSCs for regenerative cell therapies. The cell surface glycoprotein CD24 exhibits the most prominent differential expression during osteogenic versus adipogenic differentiation of human BMSCs. Therefore, CD24 may represent a selective biomarker for subpopulations of BMSCs with increased osteoblastic potential. In undifferentiated human BMSCs, CD24 cell surface expression is variable among donors (range: 2%-10%) and increased by two to fourfold upon osteogenic differentiation. Strikingly, FACS sorted CD24pos cells exhibit delayed mineralization and reduced capacity for adipocyte differentiation. RNAseq analysis of CD24pos and CD24neg BMSCs identified a limited number of genes with increased expression in CD24pos cells that are associated with cell adhesion, motility, and extracellular matrix. Downregulated genes are associated with cell cycle regulation, and biological assays revealed that CD24pos cells have reduced proliferation. Hence, expression of the cell surface glycoprotein CD24 identifies a subpopulation of human BMSCs with reduced capacity for proliferation and extracellular matrix mineralization. Functional specialization among BMSCs populations may support their regenerative potential and therapeutic success by accommodating cell activities that promote skeletal tissue formation, homeostasis, and repair.
Keywords: bone; bone marrow mesenchymal stem cells; differentiation; osteoblast.
Conflict of interest statement
No competing financial interests exist.
Figures




Similar articles
-
Foxf1 knockdown promotes BMSC osteogenesis in part by activating the Wnt/β-catenin signalling pathway and prevents ovariectomy-induced bone loss.EBioMedicine. 2020 Feb;52:102626. doi: 10.1016/j.ebiom.2020.102626. Epub 2020 Jan 22. EBioMedicine. 2020. PMID: 31981979 Free PMC article.
-
Lamc1 promotes osteogenic differentiation and inhibits adipogenic differentiation of bone marrow-derived mesenchymal stem cells.Sci Rep. 2024 Aug 23;14(1):19592. doi: 10.1038/s41598-024-69629-4. Sci Rep. 2024. PMID: 39179716 Free PMC article.
-
Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue.Stem Cell Res Ther. 2017 Dec 6;8(1):275. doi: 10.1186/s13287-017-0716-x. Stem Cell Res Ther. 2017. PMID: 29208029 Free PMC article.
-
The Role of lncRNAs in Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2020;15(3):243-249. doi: 10.2174/1574888X15666191227113742. Curr Stem Cell Res Ther. 2020. PMID: 31880266 Review.
-
Single-Cell RNA-Sequencing Reveals the Skeletal Cellular Dynamics in Bone Repair and Osteoporosis.Int J Mol Sci. 2023 Jun 6;24(12):9814. doi: 10.3390/ijms24129814. Int J Mol Sci. 2023. PMID: 37372962 Free PMC article. Review.
Cited by
-
Advances in hematopoietic stem cells ex vivo expansion associated with bone marrow niche.Ann Hematol. 2024 Dec;103(12):5035-5057. doi: 10.1007/s00277-024-05773-1. Epub 2024 Apr 30. Ann Hematol. 2024. PMID: 38684510 Review.
-
Exploring the Association Between Immune Cell Phenotypes and Osteoporosis Mediated by Inflammatory Cytokines: Insights from GWAS and Single-Cell Transcriptomics.Immunotargets Ther. 2025 Mar 17;14:227-246. doi: 10.2147/ITT.S510102. eCollection 2025. Immunotargets Ther. 2025. PMID: 40125424 Free PMC article.
-
Multiparametric senescent cell phenotyping reveals CD24 osteolineage cells as targets of senolytic therapy in the aged murine skeleton.bioRxiv [Preprint]. 2023 Jan 13:2023.01.12.523760. doi: 10.1101/2023.01.12.523760. bioRxiv. 2023. Update in: Nat Commun. 2023 Jul 31;14(1):4587. doi: 10.1038/s41467-023-40393-9. PMID: 36711531 Free PMC article. Updated. Preprint.
-
An exploration of the causal relationship between 731 immunophenotypes and osteoporosis: a bidirectional Mendelian randomized study.Front Endocrinol (Lausanne). 2024 Jul 17;15:1341002. doi: 10.3389/fendo.2024.1341002. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39086903 Free PMC article.
-
Multiparametric senescent cell phenotyping reveals targets of senolytic therapy in the aged murine skeleton.Nat Commun. 2023 Jul 31;14(1):4587. doi: 10.1038/s41467-023-40393-9. Nat Commun. 2023. PMID: 37524694 Free PMC article.
References
-
- Fernández Vallone VB, Romaniuk MA, Choi H, Labovsky V, Otaegui J and Chasseing NA. (2013). Mesenchymal stem cells and their use in therapy: what has been achieved? Differentiation 85:1–10 - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

