CD100 modulates cytotoxicity of CD8+ T cells in patients with acute myocardial infarction
- PMID: 33593275
- PMCID: PMC7888114
- DOI: 10.1186/s12865-021-00406-y
CD100 modulates cytotoxicity of CD8+ T cells in patients with acute myocardial infarction
Abstract
Background: CD100 is an immune semaphorin family member that highly expressed on T cells, which take part in the development of acute myocardial infarction (AMI). Matrix metalloproteinases (MMPs) are important mediators for membrane-bound CD100 (mCD100) shedding from T cells to generate soluble CD100 (sCD100), which has immunoregulatory effect on T cells. The aim of this study was to investigate modulatory role of CD100 on CD8+ T cell activity in AMI patients.
Methods: Peripheral sCD100 and MMP-2 level, as well as mCD100 level on T cells was assessed in patients with stable angina pectoris (SAP), unstable angina pectoris (UAP), and AMI. The regulatory function of MMP-2 on mCD100 shedding, sCD100 formation, and cytotoxicity of CD8+ T cells was analyzed in direct and indirect contact co-culture system.
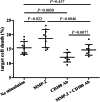
Results: AMI patients had higher peripheral sCD100 and lower mCD100 expression on CD8+ T cells in comparison with SAP, UAP, and controls. CD8+ T cells in AMI patients showed elevated direct cytotoxicity, enhanced cytokine production, and increased perforin/granzyme B secretion. Recombinant sCD100 stimulation promoted cytolytic function of CD8+ T cells in controls and AMI patients. Furthermore, AMI patients also had elevated circulating MMP-2 level. Recombinant MMP-2 stimulation induced mCD100 shedding from CD8+ T cells and sCD100 generation, resulting in enhancement of CD8+ T cell cytotoxicity in AMI patients.
Conclusion: Up-regulation of MMP-2 might contribute to elevation of mCD100 shedding and sCD100 formation, leading to increased cytotoxicity CD8+ T cells in AMI patients.
Keywords: Acute myocardial infarction; CD100; Immunoregulation; T lymphocytes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




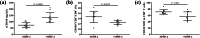
Similar articles
-
Insufficient CD100 shedding contributes to suppression of CD8+ T-cell activity in non-small cell lung cancer.Immunology. 2020 Jun;160(2):209-219. doi: 10.1111/imm.13189. Epub 2020 Apr 7. Immunology. 2020. PMID: 32149403 Free PMC article.
-
MMP2/MMP9-mediated CD100 shedding is crucial for inducing intrahepatic anti-HBV CD8 T cell responses and HBV clearance.J Hepatol. 2019 Oct;71(4):685-698. doi: 10.1016/j.jhep.2019.05.013. Epub 2019 Jun 5. J Hepatol. 2019. PMID: 31173811
-
[Ascites CD100 levels and immunomodulation effects in the peripheral blood of patients with liver cirrhosis combined with spontaneous bacterial peritonitis].Zhonghua Gan Zang Bing Za Zhi. 2023 Feb 20;31(2):138-146. doi: 10.3760/cma.j.cn501113-20220314-00108. Zhonghua Gan Zang Bing Za Zhi. 2023. PMID: 37137828 Chinese.
-
Research Advances in the Immunomodulatory Functions of CD100/SEMA4D and Their Roles in Viral Infectious Diseases.Int J Mol Sci. 2025 May 2;26(9):4341. doi: 10.3390/ijms26094341. Int J Mol Sci. 2025. PMID: 40362578 Free PMC article. Review.
-
Structure and function of the immune semaphorin CD100/SEMA4D.Crit Rev Immunol. 2003;23(1-2):65-81. doi: 10.1615/critrevimmunol.v23.i12.40. Crit Rev Immunol. 2003. PMID: 12906260 Review.
Cited by
-
Post-myocardial infarction fibrosis: Pathophysiology, examination, and intervention.Front Pharmacol. 2023 Mar 28;14:1070973. doi: 10.3389/fphar.2023.1070973. eCollection 2023. Front Pharmacol. 2023. PMID: 37056987 Free PMC article. Review.
-
Extracellular Matrix Remodeling Biomarkers in Coronary Artery Disease.Curr Top Med Chem. 2022;22(28):2355-2367. doi: 10.2174/1568026623666221024091758. Curr Top Med Chem. 2022. PMID: 36284400
-
Deciphering the role of nicotinamide metabolism and melanin-related genes in acute myocardial infarction: a machine learning approach integrating bioinformatics analysis.Korean J Physiol Pharmacol. 2025 Jul 1;29(4):521-532. doi: 10.4196/kjpp.24.431. Epub 2025 May 26. Korean J Physiol Pharmacol. 2025. PMID: 40415653 Free PMC article.
-
Bioinformatics-based Analysis and Verification of Chromatin Regulators and the Mechanism of Immune Infiltration Associated with Myocardial Infarction.Curr Med Chem. 2025;32(1):188-209. doi: 10.2174/0109298673265089231117054348. Curr Med Chem. 2025. PMID: 39354722
References
-
- Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–1866. - PubMed
-
- Wang C, Hu S, Wu J, Yu H, Pan W, Qin Y, He L, Li L, Hou J, Zhang S, et al. Characteristics and significance of healed plaques in patients with acute coronary syndrome and stable angina: an in vivo OCT and IVUS study. EuroIntervention. 2019;15(9):e771–e778. - PubMed
-
- Matusik P, Guzik B, Weber C, Guzik TJ. Do we know enough about the immune pathogenesis of acute coronary syndromes to improve clinical practice? Thromb Haemost. 2012;108(3):443–456. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

