Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis
- PMID: 33593308
- PMCID: PMC7885379
- DOI: 10.1186/s12885-021-07889-3
Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis
Abstract
Background: Radiotherapy is the mainstay of brain metastasis (BM) management. Radiation necrosis (RN) is a serious complication of radiotherapy. Bevacizumab (BV), an anti-vascular endothelial growth factor monoclonal antibody, has been increasingly used for RN treatment. We systematically reviewed the medical literature for studies reporting the efficacy and safety of bevacizumab for treatment of RN in BM patients.
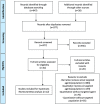
Materials and methods: PubMed, Medline, EMBASE, and Cochrane library were searched with various search keywords such as "bevacizumab" OR "anti-VEGF monoclonal antibody" AND "radiation necrosis" OR "radiation-induced brain necrosis" OR "RN" OR "RBN" AND "Brain metastases" OR "BM" until 1st Aug 2020. Studies reporting the efficacy and safety of BV treatment for BM patients with RN were retrieved. Study selection and data extraction were carried out by independent investigators. Open Meta Analyst software was used as a random effects model for meta-analysis to obtain mean reduction rates.
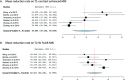
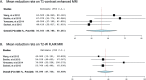
Results: Two prospective, seven retrospective, and three case report studies involving 89 patients with RN treated with BV were included in this systematic review and meta-analysis. In total, 83 (93%) patients had a recorded radiographic response to BV therapy, and six (6.7%) had experienced progressive disease. Seven studies (n = 73) reported mean volume reductions on gadolinium-enhanced T1 (mean: 47.03%, +/- 24.4) and T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI images (mean: 61.9%, +/- 23.3). Pooling together the T1 and T2 MRI reduction rates by random effects model revealed a mean of 48.58 (95% CI: 38.32-58.85) for T1 reduction rate and 62.017 (95% CI: 52.235-71.799) for T2W imaging studies. Eighty-five patients presented with neurological symptoms. After BV treatment, nine (10%) had stable symptoms, 39 (48%) had improved, and 34 (40%) patients had complete resolution of their symptoms. Individual patient data was available for 54 patients. Dexamethasone discontinuation or reduction in dosage was observed in 30 (97%) of 31 patients who had recorded dosage before and after BV treatment. Side effects were mild.
Conclusions: Bevacizumab presents a promising treatment strategy for patients with RN and brain metastatic disease. Radiographic response and clinical improvement was observed without any serious adverse events. Further class I evidence would be required to establish a bevacizumab recommendation in this group of patients.
Keywords: Adverse events; Bevacizumab (BV); Dexamethasone (Dex); MRI imaging; Radiation necrosis (RN).
Conflict of interest statement
None
Figures
References
-
- Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

