mRNA vaccine: a potential therapeutic strategy
- PMID: 33593376
- PMCID: PMC7884263
- DOI: 10.1186/s12943-021-01311-z
mRNA vaccine: a potential therapeutic strategy
Abstract
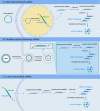
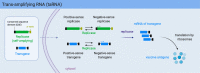
mRNA vaccines have tremendous potential to fight against cancer and viral diseases due to superiorities in safety, efficacy and industrial production. In recent decades, we have witnessed the development of different kinds of mRNAs by sequence optimization to overcome the disadvantage of excessive mRNA immunogenicity, instability and inefficiency. Based on the immunological study, mRNA vaccines are coupled with immunologic adjuvant and various delivery strategies. Except for sequence optimization, the assistance of mRNA-delivering strategies is another method to stabilize mRNAs and improve their efficacy. The understanding of increasing the antigen reactiveness gains insight into mRNA-induced innate immunity and adaptive immunity without antibody-dependent enhancement activity. Therefore, to address the problem, scientists further exploited carrier-based mRNA vaccines (lipid-based delivery, polymer-based delivery, peptide-based delivery, virus-like replicon particle and cationic nanoemulsion), naked mRNA vaccines and dendritic cells-based mRNA vaccines. The article will discuss the molecular biology of mRNA vaccines and underlying anti-virus and anti-tumor mechanisms, with an introduction of their immunological phenomena, delivery strategies, their importance on Corona Virus Disease 2019 (COVID-19) and related clinical trials against cancer and viral diseases. Finally, we will discuss the challenge of mRNA vaccines against bacterial and parasitic diseases.
Keywords: Antibody-dependent enhancement; COVID-19 mRNA vaccine; Clinical trials; Delivery strategy; Dendritic cell targeting; Immunogenicity; Modification; Non-replicating mRNA; Self-amplifying RNA; mRNA vaccine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- U19A2003/the National Natural Science Foundation Regional Innovation and Development
- No. 2018ZX09733001/National Major Scientific and Technological Special Project for "Significant New Drugs Development"
- No. 2019JDJQ008/the Excellent Youth Foundation of Sichuan Scientific Committee Grant in China
- No.2016YFA0201402/the Development Program of China
LinkOut - more resources
Full Text Sources
Other Literature Sources

