The role of pro-opiomelanocortin in the ACTH-cortisol dissociation of sepsis
- PMID: 33593393
- PMCID: PMC7885358
- DOI: 10.1186/s13054-021-03475-y
The role of pro-opiomelanocortin in the ACTH-cortisol dissociation of sepsis
Abstract
Background: Sepsis is typically hallmarked by high plasma (free) cortisol and suppressed cortisol breakdown, while plasma adrenocorticotropic hormone (ACTH) is not increased, referred to as 'ACTH-cortisol dissociation.' We hypothesized that sepsis acutely activates the hypothalamus to generate, via corticotropin-releasing hormone (CRH) and vasopressin (AVP), ACTH-induced hypercortisolemia. Thereafter, via increased availability of free cortisol, of which breakdown is reduced, feedback inhibition at the pituitary level interferes with normal processing of pro-opiomelanocortin (POMC) into ACTH, explaining the ACTH-cortisol dissociation. We further hypothesized that, in this constellation, POMC leaches into the circulation and can contribute to adrenocortical steroidogenesis.
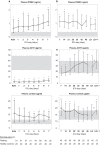
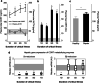
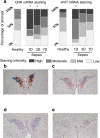
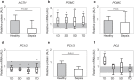
Methods: In two human studies of acute (ICU admission to day 7, N = 71) and prolonged (from ICU day 7 until recovery; N = 65) sepsis-induced critical illness, POMC plasma concentrations were quantified in relation to plasma ACTH and cortisol. In a mouse study of acute (1 day), subacute (3 and 5 days) and prolonged (7 days) fluid-resuscitated, antibiotic-treated sepsis (N = 123), we further documented alterations in hypothalamic CRH and AVP, plasma and pituitary POMC and its glucocorticoid-receptor-regulated processing into ACTH, as well as adrenal cortex integrity and steroidogenesis markers.
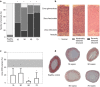
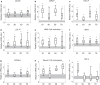
Results: The two human studies revealed several-fold elevated plasma concentrations of the ACTH precursor POMC from the acute to the prolonged phase of sepsis and upon recovery (all p < 0.0001), coinciding with the known ACTH-cortisol dissociation. Elevated plasma POMC and ACTH-corticosterone dissociation were confirmed in the mouse model. In mice, sepsis acutely increased hypothalamic mRNA of CRH (p = 0.04) and AVP (p = 0.03) which subsequently normalized. From 3 days onward, pituitary expression of CRH receptor and AVP receptor was increased. From acute throughout prolonged sepsis, pituitary POMC mRNA was always elevated (all p < 0.05). In contrast, markers of POMC processing into ACTH and of ACTH secretion, negatively regulated by glucocorticoid receptor ligand binding, were suppressed at all time points (all p ≤ 0.05). Distorted adrenocortical structure (p < 0.05) and lipid depletion (p < 0.05) were present, while most markers of adrenocortical steroidogenic activity were increased at all time points (all p < 0.05).
Conclusion: Together, these findings suggest that increased circulating POMC, through CRH/AVP-driven POMC expression and impaired processing into ACTH, could represent a new piece in the puzzling ACTH-cortisol dissociation.
Keywords: Adrenal; Adrenocorticotropic hormone; Glucocorticoid receptor; Pituitary; Pro-opiomelanocortin; Sepsis.
Conflict of interest statement
All authors declare that there is no conflict of interest to report.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

