Coexistence of the Entner-Doudoroff and Embden-Meyerhof-Parnas pathways enhances glucose consumption of ethanol-producing Corynebacterium glutamicum
- PMID: 33593398
- PMCID: PMC7888142
- DOI: 10.1186/s13068-021-01876-3
Coexistence of the Entner-Doudoroff and Embden-Meyerhof-Parnas pathways enhances glucose consumption of ethanol-producing Corynebacterium glutamicum
Abstract
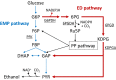
Background: It is interesting to modify sugar metabolic pathways to improve the productivity of biocatalysts that convert sugars to value-added products. However, this attempt often fails due to the tight control of the sugar metabolic pathways. Recently, activation of the Entner-Doudoroff (ED) pathway in Escherichia coli has been shown to enhance glucose consumption, though the mechanism underlying this phenomenon is poorly understood. In the present study, we investigated the effect of a functional ED pathway in metabolically engineered Corynebacterium glutamicum that metabolizes glucose via the Embden-Meyerhof-Parnas (EMP) pathway to produce ethanol under oxygen deprivation. This study aims to provide further information on metabolic engineering strategies that allow the Entner-Doudoroff and Embden-Meyerhof-Parnas pathways to coexist.
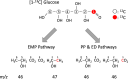
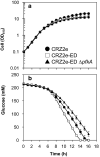
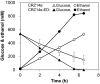
Results: Three genes (zwf, edd, and eda) encoding glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydratase, and 2-keto-3-deoxy-6-phosphogluconate aldolase from Zymomonas mobilis were expressed in a genetically modified strain, C. glutamicum CRZ2e, which produces pyruvate decarboxylase and alcohol dehydrogenase from Z. mobilis. A 13C-labeling experiment using [1-13C] glucose indicated a distinctive 13C distribution of ethanol between the parental and the ED-introduced strains, which suggested an alteration of carbon flux as a consequence of ED pathway introduction. The ED-introduced strain, CRZ2e-ED, consumed glucose 1.5-fold faster than the parental strain. A pfkA deletion mutant of CRZ2e-ED (CRZ2e-EDΔpfkA) was also constructed to evaluate the effects of EMP pathway inactivation, which showed an almost identical rate of glucose consumption compared to that of the parental CRZ2e strain. The introduction of the ED pathway did not alter the intracellular NADH/NAD+ ratio, whereas it resulted in a slight increase in the ATP/ADP ratio. The recombinant strains with simultaneous overexpression of the genes for the EMP and ED pathways exhibited the highest ethanol productivity among all C. glutamicum strains ever constructed.
Conclusions: The increased sugar consumption observed in ED-introduced strains was not a consequence of cofactor balance alterations, but rather the crucial coexistence of two active glycolytic pathways for enhanced glucose consumption. Coexistence of the ED and EMP pathways is a good strategy for improving biocatalyst productivity even when NADPH supply is not a limiting factor for fermentation.
Keywords: Corynebacterium glutamicum; Entner–Doudoroff pathway; Glycolysis; Oxygen deprivation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

