The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1
- PMID: 33593403
- PMCID: PMC7885589
- DOI: 10.1186/s13045-021-01045-x
The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1
Abstract
Background: Therapeutic antibodies targeting programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) axis induce potent and durable anti-tumor responses in multiple types of cancers. However, only a subset of patients benefits from anti-PD-1/PD-L1 therapies. As a negative regulator of anti-tumor immunity, TGF-β impairs the efficacy of anti-PD-1/PD-L1 and induces drug resistance. Developing a novel treatment strategy to simultaneously block PD-1/PD-L1 and TGF-β would be valuable to enhance the effect of anti-PD-1/PD-L1 and relieve drug resistance.
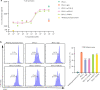
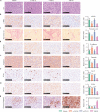
Methods: Based on the Check-BODY™ technology platform, we developed an anti-TGF-β/PD-L1 bispecific antibody YM101. The bioactivity of the anti-TGF-β moiety was determined by Smad-luciferase reporter assay, transwell assay, western blotting, CCK-8, and flow cytometry. The bioactivity of the anti-PD-L1 moiety was measured by T cell activation assays. EMT-6, CT26, and 3LL tumor models were used to investigate the anti-tumor activity of YM101 in vivo. RNA-seq, immunohistochemical staining, and flow cytometry were utilized to analyze the effect of YM101 on the tumor microenvironment.
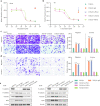
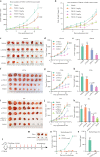
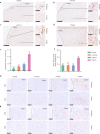
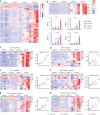
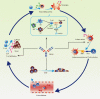
Results: YM101 could bind to TGF-β and PD-L1 specifically. In vitro experiments showed that YM101 effectively counteracted the biological effects of TGF-β and PD-1/PD-L1 pathway, including activating Smad signaling, inducing epithelial-mesenchymal transition, and immunosuppression. Besides, in vivo experiments indicated the anti-tumor activity of YM101 was superior to anti-TGF-β and anti-PD-L1 monotherapies. Mechanistically, YM101 promoted the formation of 'hot tumor': increasing the numbers of tumor infiltrating lymphocytes and dendritic cells, elevating the ratio of M1/M2, and enhancing cytokine production in T cells. This normalized tumor immune microenvironment and enhanced anti-tumor immune response might contribute to the robust anti-tumor effect of YM101.
Conclusion: Our results demonstrated that YM101 could simultaneously block TGF-β and PD-L1 pathways and had a superior anti-tumor effect compared to the monotherapies.
Keywords: Bispecific antibody; Cancer immunotherapy; Immune checkpoint; Immune normalization; PD-1; PD-L1; TGF-β; The tumor microenvironment.
Conflict of interest statement
JZ, YY, and PZ were employees of Wuhan YZY Biopharma Co., Ltd.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

