Comparison of acute pneumonia caused by SARS-COV-2 and other respiratory viruses in children: a retrospective multi-center cohort study during COVID-19 outbreak
- PMID: 33593415
- PMCID: PMC7886299
- DOI: 10.1186/s40779-021-00306-7
Comparison of acute pneumonia caused by SARS-COV-2 and other respiratory viruses in children: a retrospective multi-center cohort study during COVID-19 outbreak
Abstract
Background: Until January 18, 2021, coronavirus disease-2019 (COVID-19) has infected more than 93 million individuals and has caused a certain degree of panic. Viral pneumonia caused by common viruses such as respiratory syncytial virus, rhinovirus, human metapneumovirus, human bocavirus, and parainfluenza viruses have been more common in children. However, the incidence of COVID-19 in children was significantly lower than that in adults. The purpose of this study was to describe the clinical manifestations, treatment and outcomes of COVID-19 in children compared with those of other sources of viral pneumonia diagnosed during the COVID-19 outbreak.
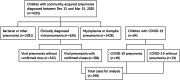
Methods: Children with COVID-19 and viral pneumonia admitted to 20 hospitals were enrolled in this retrospective multi-center cohort study. A total of 64 children with COVID-19 were defined as the COVID-19 cohort, of which 40 children who developed pneumonia were defined as the COVID-19 pneumonia cohort. Another 284 children with pneumonia caused by other viruses were defined as the viral pneumonia cohort. The epidemiologic, clinical, and laboratory findings were compared by Kolmogorov-Smirnov test, t-test, Mann-Whitney U test and Contingency table method. Drug usage, immunotherapy, blood transfusion, and need for oxygen support were collected as the treatment indexes. Mortality, intensive care needs and symptomatic duration were collected as the outcome indicators.
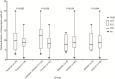
Results: Compared with the viral pneumonia cohort, children in the COVID-19 cohort were mostly exposed to family members confirmed to have COVID-19 (53/64 vs. 23/284), were of older median age (6.3 vs. 3.2 years), and had a higher proportion of ground-glass opacity (GGO) on computed tomography (18/40 vs. 0/38, P < 0.001). Children in the COVID-19 pneumonia cohort had a lower proportion of severe cases (1/40 vs. 38/284, P = 0.048), and lower cases with high fever (3/40 vs. 167/284, P < 0.001), requiring intensive care (1/40 vs. 32/284, P < 0.047) and with shorter symptomatic duration (median 5 vs. 8 d, P < 0.001). The proportion of cases with evaluated inflammatory indicators, biochemical indicators related to organ or tissue damage, D-dimer and secondary bacterial infection were lower in the COVID-19 pneumonia cohort than those in the viral pneumonia cohort (P < 0.05). No statistical differences were found in the duration of positive PCR results from pharyngeal swabs in 25 children with COVID-19 who received antiviral drugs (lopinavir-ritonavir, ribavirin, and arbidol) as compared with duration in 39 children without antiviral therapy [median 10 vs. 9 d, P = 0.885].
Conclusion: The symptoms and severity of COVID-19 pneumonia in children were no more severe than those in children with other viral pneumonia. Lopinavir-ritonavir, ribavirin and arbidol do not shorten the duration of positive PCR results from pharyngeal swabs in children with COVID-19. During the COVID-19 outbreak, attention also must be given to children with infection by other pathogens infection.
Keywords: COVID-19; Children; Severe acute respiratory syndrome; Viral pneumonia.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain.Crit Care. 2020 Nov 26;24(1):666. doi: 10.1186/s13054-020-03332-4. Crit Care. 2020. PMID: 33243303 Free PMC article.
-
[Investigation of pathogenic agents causing acute respiratory tract infections in pediatric patients in a children's hospital assigned for case screening in Beijing during the outbreak of COVID-19].Zhonghua Er Ke Za Zhi. 2020 Aug 2;58(8):635-639. doi: 10.3760/cma.j.cn112140-20200426-00437. Zhonghua Er Ke Za Zhi. 2020. PMID: 32842383 Chinese.
-
Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore.JAMA. 2020 Apr 21;323(15):1488-1494. doi: 10.1001/jama.2020.3204. JAMA. 2020. PMID: 32125362 Free PMC article.
-
Atypical Manifestations of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children: A Systematic Review.Curr Pediatr Rev. 2021;17(3):162-171. doi: 10.2174/1573396317666210406153302. Curr Pediatr Rev. 2021. PMID: 33823780
-
Clinical features of COVID-19 and SARS epidemics. A literature review.J Prev Med Hyg. 2021 Apr 29;62(1):E13-E24. doi: 10.15167/2421-4248/jpmh2021.62.1.1680. eCollection 2021 Mar. J Prev Med Hyg. 2021. PMID: 34322612 Free PMC article. Review.
Cited by
-
Current utilization of interferon alpha for the treatment of coronavirus disease 2019: A comprehensive review.Cytokine Growth Factor Rev. 2022 Feb;63:34-43. doi: 10.1016/j.cytogfr.2022.01.001. Epub 2022 Jan 13. Cytokine Growth Factor Rev. 2022. PMID: 35115233 Free PMC article. Review.
-
Respiratory Syncytial Virus: A Systematic Review and Meta-Analysis of Tomographic Findings (2000-2022).Children (Basel). 2023 Jul 5;10(7):1169. doi: 10.3390/children10071169. Children (Basel). 2023. PMID: 37508666 Free PMC article. Review.
-
Coronavirus Disease 2019 in Children.Front Pediatr. 2021 May 28;9:668484. doi: 10.3389/fped.2021.668484. eCollection 2021. Front Pediatr. 2021. PMID: 34123972 Free PMC article. Review.
-
Children and Adults in a Household Cohort Study Have Robust Longitudinal Immune Responses Following SARS-CoV-2 Infection or Exposure.Front Immunol. 2021 Oct 13;12:741639. doi: 10.3389/fimmu.2021.741639. eCollection 2021. Front Immunol. 2021. PMID: 34721408 Free PMC article.
-
Effect of Fu Zheng Jie Du Formula on outcomes in patients with severe pneumonia receiving prone ventilation: a retrospective cohort study.Front Pharmacol. 2024 Jul 24;15:1428817. doi: 10.3389/fphar.2024.1428817. eCollection 2024. Front Pharmacol. 2024. PMID: 39114366 Free PMC article.
References
-
- Cauchemez S, Fraser C, Van Kerkhove MD, Donnelly CA, Riley S, Rambaut A, et al. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect Dis. 2014;14(1):50–56. doi: 10.1016/S1473-3099(13)70304-9. - DOI - PMC - PubMed
-
- Who. WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/. Accessed 18 Jan 2021.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

