Modulating gut microbiota in a mouse model of Graves' orbitopathy and its impact on induced disease
- PMID: 33593429
- PMCID: PMC7888139
- DOI: 10.1186/s40168-020-00952-4
Modulating gut microbiota in a mouse model of Graves' orbitopathy and its impact on induced disease
Abstract
Background: Graves' disease (GD) is an autoimmune condition in which autoantibodies to the thyrotropin receptor (TSHR) cause hyperthyroidism. About 50% of GD patients also have Graves' orbitopathy (GO), an intractable disease in which expansion of the orbital contents causes diplopia, proptosis and even blindness. Murine models of GD/GO, developed in different centres, demonstrated significant variation in gut microbiota composition which correlated with TSHR-induced disease heterogeneity. To investigate whether correlation indicates causation, we modified the gut microbiota to determine whether it has a role in thyroid autoimmunity. Female BALB/c mice were treated with either vancomycin, probiotic bacteria, human fecal material transfer (hFMT) from patients with severe GO or ddH2O from birth to immunization with TSHR-A subunit or beta-galactosidase (βgal; age ~ 6 weeks). Incidence and severity of GD (TSHR autoantibodies, thyroid histology, thyroxine level) and GO (orbital fat and muscle histology), lymphocyte phenotype, cytokine profile and gut microbiota were analysed at sacrifice (~ 22 weeks).
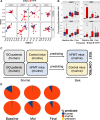
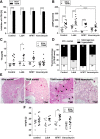
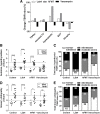
Results: In ddH2O-TSHR mice, 84% had pathological autoantibodies, 67% elevated thyroxine, 77% hyperplastic thyroids and 70% orbital pathology. Firmicutes were increased, and Bacteroidetes reduced relative to ddH2O-βgal; CCL5 was increased. The random forest algorithm at the genus level predicted vancomycin treatment with 100% accuracy but 74% and 70% for hFMT and probiotic, respectively. Vancomycin significantly reduced gut microbiota richness and diversity compared with all other groups; the incidence and severity of both GD and GO also decreased; reduced orbital pathology correlated positively with Akkermansia spp. whilst IL-4 levels increased. Mice receiving hFMT initially inherited their GO donors' microbiota, and the severity of induced GD increased, as did the orbital brown adipose tissue volume in TSHR mice. Furthermore, genus Bacteroides, which is reduced in GD patients, was significantly increased by vancomycin but reduced in hFMT-treated mice. Probiotic treatment significantly increased CD25+ Treg cells in orbital draining lymph nodes but exacerbated induced autoimmune hyperthyroidism and GO.
Conclusions: These results strongly support a role for the gut microbiota in TSHR-induced disease. Whilst changes to the gut microbiota have a profound effect on quantifiable GD endocrine and immune factors, the impact on GO cellular changes is more nuanced. The findings have translational potential for novel, improved treatments. Video abstract.
Keywords: Graves’ disease; Graves’ orbitopathy; Gut microbiota; Human fecal microbiota transplant; Microbiome modulation; Murine model; Probiotics; Vancomycin.
Conflict of interest statement
HLV, DC, SP and DM are/were employees of Cultech Ltd. JRM and GM are involved in other collaborative projects with Cultech Ltd. The other authors declare that they have no competing interests.
Figures



Similar articles
-
Gut microbiota in experimental murine model of Graves' orbitopathy established in different environments may modulate clinical presentation of disease.Microbiome. 2018 May 25;6(1):97. doi: 10.1186/s40168-018-0478-4. Microbiome. 2018. PMID: 29801507 Free PMC article.
-
The role and molecular mechanism of gut microbiota in Graves' orbitopathy.J Endocrinol Invest. 2023 Feb;46(2):305-317. doi: 10.1007/s40618-022-01902-7. Epub 2022 Aug 20. J Endocrinol Invest. 2023. PMID: 35986869
-
The Role of the Microbiota in Graves' Disease and Graves' Orbitopathy.Front Cell Infect Microbiol. 2021 Dec 22;11:739707. doi: 10.3389/fcimb.2021.739707. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 35004341 Free PMC article. Review.
-
A Promising Mouse Model of Graves' Orbitopathy Induced by Adenovirus Expressing Thyrotropin Receptor A Subunit.Thyroid. 2021 Apr;31(4):638-648. doi: 10.1089/thy.2020.0088. Epub 2021 Jan 5. Thyroid. 2021. PMID: 33076782
-
Thyrotropin receptor antibodies and Graves' orbitopathy.J Endocrinol Invest. 2021 Apr;44(4):703-712. doi: 10.1007/s40618-020-01380-9. Epub 2020 Aug 4. J Endocrinol Invest. 2021. PMID: 32749654 Free PMC article. Review.
Cited by
-
The relationships between the gut microbiota and its metabolites with thyroid diseases.Front Endocrinol (Lausanne). 2022 Aug 18;13:943408. doi: 10.3389/fendo.2022.943408. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060978 Free PMC article. Review.
-
Bacteroides acidifaciens in the gut plays a protective role against CD95-mediated liver injury.Gut Microbes. 2022 Jan-Dec;14(1):2027853. doi: 10.1080/19490976.2022.2027853. Gut Microbes. 2022. PMID: 35129072 Free PMC article.
-
Linsitinib, an IGF-1R inhibitor, attenuates disease development and progression in a model of thyroid eye disease.Front Endocrinol (Lausanne). 2023 Jun 26;14:1211473. doi: 10.3389/fendo.2023.1211473. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37435490 Free PMC article.
-
Gut microbial contributions to thermogenesis.J Exp Biol. 2025 Jul 15;228(14):jeb249791. doi: 10.1242/jeb.249791. Epub 2025 Jul 11. J Exp Biol. 2025. PMID: 40642960 Review.
-
Unveiling the Role of Gut Microbiota and Metabolites in Autoimmune Thyroid Diseases: Emerging Perspectives.Int J Mol Sci. 2024 Oct 10;25(20):10918. doi: 10.3390/ijms252010918. Int J Mol Sci. 2024. PMID: 39456701 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

