A new candidate vaccine for human brucellosis based on influenza viral vectors: a preliminary investigation for the development of an immunization schedule in a guinea pig model
- PMID: 33593447
- PMCID: PMC7886305
- DOI: 10.1186/s40249-021-00801-y
A new candidate vaccine for human brucellosis based on influenza viral vectors: a preliminary investigation for the development of an immunization schedule in a guinea pig model
Abstract
Background: A new candidate vector vaccine against human brucellosis based on recombinant influenza viral vectors (rIVV) subtypes H5N1 expressing Brucella outer membrane protein (Omp) 16, L7/L12, Omp19 or Cu-Zn SOD proteins has been developed. This paper presents the results of the study of protection of the vaccine using on guinea pigs, including various options of administering, dose and frequency. Provided data of the novel vaccine candidate will contribute to its further movement into the preclinical stage study.
Methods: General states of guinea pigs was assessed based on behavior and dynamics of a guinea pig weight-gain test. The effectiveness of the new anti-brucellosis vector vaccine was determined by studying its protective effect after conjunctival, intranasal and sublingual administration in doses 105 EID50, 106 EID50 and 107 EID50 during prime and boost vaccinations of animals, followed by challenge with a virulent strain of B. melitensis 16 M infection. For sake of comparison, the commercial B. melitensis Rev.1 vaccine was used as a control. The protective properties of vaccines were assessed by quantitation of Brucella colonization in organs and tissues of infected animals and compared to the control groups.
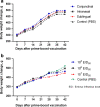
Results: It was observed a gradual increase in body weight of guinea pigs after prime and booster immunization with the vaccine using conjunctival, intranasal and sublingual routes of administration, as well as after using various doses of vaccine. The most optimal way of using the vaccine has been established: double intranasal immunization of guinea pigs at a dose of 106 EID50, which provides 80% protection of guinea pigs from B. melitensis 16 M infection (P < 0.05), which is comparable to the results of the effectiveness of the commercial B. melitensis Rev.1 vaccine.
Conclusions: We developed effective human vaccine candidate against brucellosis and developed its immunization protocol in guinea pig model. We believe that because of these studies, the proposed vaccine has achieved the best level of protection, which in turn provides a basis for its further promotion.
Keywords: Guinea pigs; Human brucellosis; Immunization route; Influenza viral vectors; Protection; Vaccination dose; Vaccine candidate.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


Similar articles
-
Development of Human Vectored Brucellosis Vaccine Formulation: Assessment of Safety and Protectiveness of Influenza Viral Vectors Expressing Brucella Immunodominant Proteins in Mice and Guinea Pigs.Biomed Res Int. 2020 Nov 19;2020:1438928. doi: 10.1155/2020/1438928. eCollection 2020. Biomed Res Int. 2020. PMID: 33274194 Free PMC article.
-
Influenza viral vectors expressing the Brucella OMP16 or L7/L12 proteins as vaccines against B. abortus infection.Virol J. 2014 Apr 10;11:69. doi: 10.1186/1743-422X-11-69. Virol J. 2014. PMID: 24716528 Free PMC article.
-
Improved influenza viral vector based Brucella abortus vaccine induces robust B and T-cell responses and protection against Brucella melitensis infection in pregnant sheep and goats.PLoS One. 2017 Oct 12;12(10):e0186484. doi: 10.1371/journal.pone.0186484. eCollection 2017. PLoS One. 2017. PMID: 29023541 Free PMC article.
-
A review of the use of B. melitensis Rev 1 vaccine in adult sheep and goats.Prev Vet Med. 1997 Aug;31(3-4):275-83. doi: 10.1016/s0167-5877(96)01110-5. Prev Vet Med. 1997. PMID: 9234451 Review.
-
Laboratory animal models for brucellosis research.J Biomed Biotechnol. 2011;2011:518323. doi: 10.1155/2011/518323. Epub 2011 Feb 20. J Biomed Biotechnol. 2011. PMID: 21403904 Free PMC article. Review.
Cited by
-
Peptide-Based Vaccines for Tuberculosis.Front Immunol. 2022 Jan 31;13:830497. doi: 10.3389/fimmu.2022.830497. eCollection 2022. Front Immunol. 2022. PMID: 35173740 Free PMC article. Review.
-
Prophylactic vaccine delivery systems against epidemic infectious diseases.Adv Drug Deliv Rev. 2021 Sep;176:113867. doi: 10.1016/j.addr.2021.113867. Epub 2021 Jul 17. Adv Drug Deliv Rev. 2021. PMID: 34280513 Free PMC article. Review.
-
Registered Influenza Viral Vector Based Brucella abortus Vaccine for Cattle in Kazakhstan: Age-Wise Safety and Efficacy Studies.Front Cell Infect Microbiol. 2021 Jul 1;11:669196. doi: 10.3389/fcimb.2021.669196. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34290993 Free PMC article.
-
Evaluation of Brucellosis Vaccines: A Comprehensive Review.Front Vet Sci. 2022 Jul 18;9:925773. doi: 10.3389/fvets.2022.925773. eCollection 2022. Front Vet Sci. 2022. PMID: 35923818 Free PMC article. Review.
-
Brucella osteoarthritis: recent progress and future directions.Front Microbiol. 2025 Feb 4;16:1522537. doi: 10.3389/fmicb.2025.1522537. eCollection 2025. Front Microbiol. 2025. PMID: 39967734 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

