Diversity and Function of Glial Cell Types in Multiple Sclerosis
- PMID: 33593693
- PMCID: PMC7914214
- DOI: 10.1016/j.it.2021.01.005
Diversity and Function of Glial Cell Types in Multiple Sclerosis
Abstract
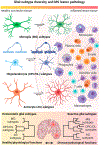
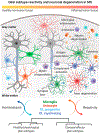
Glial subtype diversity is an emerging topic in neurobiology and immune-mediated neurological diseases such as multiple sclerosis (MS). We discuss recent conceptual and technological advances that allow a better understanding of the transcriptomic and functional heterogeneity of oligodendrocytes (OLs), astrocytes, and microglial cells under inflammatory-demyelinating conditions. Recent single cell transcriptomic studies suggest the occurrence of novel homeostatic and reactive glial subtypes and provide insight into the molecular events during disease progression. Multiplexed RNA in situ hybridization has enabled 'mapping back' dysregulated gene expression to glial subtypes within the MS lesion microenvironment. These findings suggest novel homeostatic and reactive glial-cell-type functions both in immune-related processes and neuroprotection relevant to understanding the pathology of MS.
Keywords: astrocytes; microglia; multiple sclerosis; neuroinflammation; oligodendrocytes; transcriptomics.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors have no interests to declare.
Figures


Similar articles
-
Cross-regional homeostatic and reactive glial signatures in multiple sclerosis.Acta Neuropathol. 2022 Nov;144(5):987-1003. doi: 10.1007/s00401-022-02497-2. Epub 2022 Sep 16. Acta Neuropathol. 2022. PMID: 36112223 Free PMC article.
-
Multiple Sclerosis: Glial Cell Diversity in Time and Space.Glia. 2025 Mar;73(3):574-590. doi: 10.1002/glia.24655. Epub 2024 Dec 24. Glia. 2025. PMID: 39719685 Free PMC article. Review.
-
Comparing RNA-sequencing datasets from astrocytes, oligodendrocytes, and microglia in multiple sclerosis identifies novel dysregulated genes relevant to inflammation and myelination.WIREs Mech Dis. 2023 Mar;15(2):e1594. doi: 10.1002/wsbm.1594. Epub 2023 Jan 4. WIREs Mech Dis. 2023. PMID: 36600404 Review.
-
The role of glial cells in multiple sclerosis disease progression.Nat Rev Neurol. 2022 Apr;18(4):237-248. doi: 10.1038/s41582-022-00624-x. Epub 2022 Feb 21. Nat Rev Neurol. 2022. PMID: 35190704 Review.
-
Glial Cells as Key Regulators in Neuroinflammatory Mechanisms Associated with Multiple Sclerosis.Int J Mol Sci. 2024 Sep 4;25(17):9588. doi: 10.3390/ijms25179588. Int J Mol Sci. 2024. PMID: 39273535 Free PMC article. Review.
Cited by
-
Editorial: Gliopathies in aging-related brain diseases: From understanding to therapy.Front Neurosci. 2022 Sep 14;16:1017877. doi: 10.3389/fnins.2022.1017877. eCollection 2022. Front Neurosci. 2022. PMID: 36188470 Free PMC article. No abstract available.
-
Cell-specific NFIA upregulation promotes epileptogenesis by TRPV4-mediated astrocyte reactivity.J Neuroinflammation. 2023 Oct 25;20(1):247. doi: 10.1186/s12974-023-02909-4. J Neuroinflammation. 2023. PMID: 37880726 Free PMC article.
-
Contribution of Oligodendrocytes, Microglia, and Astrocytes to Myelin Debris Uptake in an Explant Model of Inflammatory Demyelination in Rats.Cells. 2023 Sep 3;12(17):2203. doi: 10.3390/cells12172203. Cells. 2023. PMID: 37681935 Free PMC article.
-
Neurodegeneration and demyelination in multiple sclerosis.Neuron. 2024 Oct 9;112(19):3231-3251. doi: 10.1016/j.neuron.2024.05.025. Epub 2024 Jun 17. Neuron. 2024. PMID: 38889714 Review.
-
Shining the Light on Astrocytic Ensembles.Cells. 2023 Apr 26;12(9):1253. doi: 10.3390/cells12091253. Cells. 2023. PMID: 37174653 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

