Population differences in vaccine responses (POPVAC): scientific rationale and cross-cutting analyses for three linked, randomised controlled trials assessing the role, reversibility and mediators of immunomodulation by chronic infections in the tropics
- PMID: 33593767
- PMCID: PMC7893603
- DOI: 10.1136/bmjopen-2020-040425
Population differences in vaccine responses (POPVAC): scientific rationale and cross-cutting analyses for three linked, randomised controlled trials assessing the role, reversibility and mediators of immunomodulation by chronic infections in the tropics
Abstract
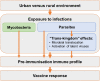
Introduction: Vaccine-specific immune responses vary between populations and are often impaired in low income, rural settings. Drivers of these differences are not fully elucidated, hampering identification of strategies for optimising vaccine effectiveness. We hypothesise that urban-rural (and regional and international) differences in vaccine responses are mediated to an important extent by differential exposure to chronic infections, particularly parasitic infections.
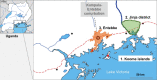
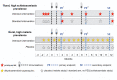
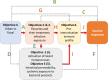
Methods and analysis: Three related trials sharing core elements of study design and procedures (allowing comparison of outcomes across the trials) will test the effects of (1) individually randomised intervention against schistosomiasis (trial A) and malaria (trial B), and (2) Bacillus Calmette-Guérin (BCG) revaccination (trial C), on a common set of vaccine responses. We will enrol adolescents from Ugandan schools in rural high-schistosomiasis (trial A) and rural high-malaria (trial B) settings and from an established urban birth cohort (trial C). All participants will receive BCG on day '0'; yellow fever, oral typhoid and human papilloma virus (HPV) vaccines at week 4; and HPV and tetanus/diphtheria booster vaccine at week 28. Primary outcomes are BCG-specific IFN-γ responses (8 weeks after BCG) and for other vaccines, antibody responses to key vaccine antigens at 4 weeks after immunisation. Secondary analyses will determine effects of interventions on correlates of protective immunity, vaccine response waning, priming versus boosting immunisations, and parasite infection status and intensity. Overarching analyses will compare outcomes between the three trial settings. Sample archives will offer opportunities for exploratory evaluation of the role of immunological and 'trans-kingdom' mediators in parasite modulation of vaccine-specific responses.
Ethics and dissemination: Ethics approval has been obtained from relevant Ugandan and UK ethics committees. Results will be shared with Uganda Ministry of Health, relevant district councils, community leaders and study participants. Further dissemination will be done through conference proceedings and publications.
Trial registration numbers: ISRCTN60517191, ISRCTN62041885, ISRCTN10482904.
Keywords: epidemiology; immunology; infection control; paediatric infectious disease & immunisation; parasitology; public health.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: AME reports a grant from the Medical research Council, UK (POPVAC programme funding). The rest of the authors declare that they have no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
