Effect of intensive treatment for schistosomiasis on immune responses to vaccines among rural Ugandan island adolescents: randomised controlled trial protocol A for the ' POP ulation differences in VAC cine responses' (POPVAC) programme
- PMID: 33593768
- PMCID: PMC7888376
- DOI: 10.1136/bmjopen-2020-040426
Effect of intensive treatment for schistosomiasis on immune responses to vaccines among rural Ugandan island adolescents: randomised controlled trial protocol A for the ' POP ulation differences in VAC cine responses' (POPVAC) programme
Abstract
Introduction: Several licensed and investigational vaccines have lower efficacy, and induce impaired immune responses, in low-income versus high-income countries and in rural, versus urban, settings. Understanding these population differences is essential to optimising vaccine effectiveness in the tropics. We suggest that repeated exposure to and immunomodulation by chronic helminth infections partly explains population differences in vaccine response.
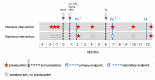
Methods and analysis: We have designed an individually randomised, parallel group trial of intensive versus standard praziquantel (PZQ) intervention against schistosomiasis, to determine effects on vaccine response outcomes among school-going adolescents (9-17 years) from rural Schistosoma mansoni-endemic Ugandan islands. Vaccines to be studied comprise BCG on day 'zero'; yellow fever, oral typhoid and human papilloma virus (HPV) vaccines at week 4; and HPV and tetanus/diphtheria booster vaccine at week 28. The intensive arm will receive PZQ doses three times, each 2 weeks apart, before BCG immunisation, followed by a dose at week 8 and quarterly thereafter. The standard arm will receive PZQ at week 8 and 52. We expect to enrol 480 participants, with 80% infected with S. mansoni at the outset.Primary outcomes are BCG-specific interferon-γ ELISpot responses 8 weeks after BCG immunisation and for other vaccines, antibody responses to key vaccine antigens at 4 weeks after immunisation. Secondary analyses will determine the effects of intensive anthelminthic treatment on correlates of protective immunity, on waning of vaccine response, on priming versus boosting immunisations and on S. mansoni infection status and intensity. Exploratory immunology assays using archived samples will enable assessment of mechanistic links between helminths and vaccine responses.
Ethics and dissemination: Ethics approval has been obtained from relevant ethics committes of Uganda and UK. Results will be shared with Uganda Ministry of Health, relevant district councils, community leaders and study participants. Further dissemination will be done through conference proceedings and publications.
Trial registration number: ISRCTN60517191.
Keywords: epidemiology; immunology; infection control; paediatric infectious disease & immunisation; parasitology; public health.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: Alison Elliott reports a grant from the Medical research Council, UK (POPVAC programme funding). The rest of the authors declare that they have no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources