Therapeutic Targeting of DGKA-Mediated Macropinocytosis Leads to Phospholipid Reprogramming in Tuberous Sclerosis Complex
- PMID: 33593821
- PMCID: PMC8137542
- DOI: 10.1158/0008-5472.CAN-20-2218
Therapeutic Targeting of DGKA-Mediated Macropinocytosis Leads to Phospholipid Reprogramming in Tuberous Sclerosis Complex
Abstract
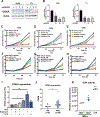
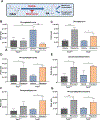
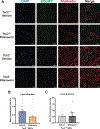
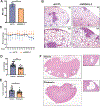
Lymphangioleiomyomatosis is a rare destructive lung disease affecting primarily women and is the primary lung manifestation of tuberous sclerosis complex (TSC). In lymphangioleiomyomatosis, biallelic loss of TSC1/2 leads to hyperactivation of mTORC1 and inhibition of autophagy. To determine how the metabolic vulnerabilities of TSC2-deficient cells can be targeted, we performed a high-throughput screen utilizing the "Repurposing" library at the Broad Institute of MIT and Harvard (Cambridge, MA), with or without the autophagy inhibitor chloroquine. Ritanserin, an inhibitor of diacylglycerol kinase alpha (DGKA), was identified as a selective inhibitor of proliferation of Tsc2-/- mouse embryonic fibroblasts (MEF), with no impact on Tsc2+/+ MEFs. DGKA is a lipid kinase that metabolizes diacylglycerol to phosphatidic acid, a key component of plasma membranes. Phosphatidic acid levels were increased 5-fold in Tsc2-/- MEFs compared with Tsc2+/+ MEFs, and treatment of Tsc2-/- MEFs with ritanserin led to depletion of phosphatidic acid as well as rewiring of phospholipid metabolism. Macropinocytosis is known to be upregulated in TSC2-deficient cells. Ritanserin decreased macropinocytic uptake of albumin, limited the number of lysosomes, and reduced lysosomal activity in Tsc2-/- MEFs. In a mouse model of TSC, ritanserin treatment decreased cyst frequency and volume, and in a mouse model of lymphangioleiomyomatosis, genetic downregulation of DGKA prevented alveolar destruction and airspace enlargement. Collectively, these data indicate that DGKA supports macropinocytosis in TSC2-deficient cells to maintain phospholipid homeostasis and promote proliferation. Targeting macropinocytosis with ritanserin may represent a novel therapeutic approach for the treatment of TSC and lymphangioleiomyomatosis. SIGNIFICANCE: This study identifies macropinocytosis and phospholipid metabolism as novel mechanisms of metabolic homeostasis in mTORC1-hyperactive cells and suggest ritanserin as a novel therapeutic strategy for use in mTORC1-hyperactive tumors, including pancreatic cancer. GRAPHICAL ABSTRACT: http://cancerres.aacrjournals.org/content/canres/81/8/2086/F1.large.jpg.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

